Explain why one of these compounds reacts readily by an E2 mechanism when treated with sodium ethoxide
Question:
Explain why one of these compounds reacts readily by an E2 mechanism when treated with sodium ethoxide in ethanol but the other doesnot:
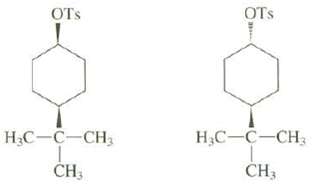
Transcribed Image Text:
OTs OTS Н.С— С—СH, Н.С—С—СH, CH; CH3 зн
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
The bulky tBu group must be equatorial and locks the ring in a single conformation For e...View the full answer

Answered By

Douglas Makokha
Unlock Academic Success with Dedicated Tutoring and Expert Writing Support!
Are you ready to excel in your academics? Look no further! As a passionate tutor, I believe that dedication and hard work are the keys to achieving outstanding results. When it comes to academics, I strive to provide nothing but the best for every student I encounter.
With a relentless thirst for knowledge, I have extensively researched numerous subjects and topics, equipping myself with a treasure trove of answers to tackle any question that comes my way. With four years of invaluable experience, I have mastered the art of unraveling even the most intricate problems. Collaborating with esteemed writers has granted me exclusive access to the trade secrets utilized by the industry's top professionals.
Allow me the pleasure of assisting you with your writing assignments. I thrive on challenges and will guide you through any obstacles you may face. Together, we will unlock your academic potential and pave the way for your success.
4.90+
62+ Reviews
349+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
When cis-1-bromo-4-tert-butylcyclohexane is treated with sodium ethoxide in ethanol, it reacts rapidly; the product is 4-tert-butylcyclohexene. Under the same conditions, trans-...
-
The reaction between 2-bromobutane and sodium ethoxide in ethanol gives rise to three E2 products. What are they? Predict their relative amounts.
-
The reaction in eq. 7.17 occurs by an E2 mechanism (review eqs. 7.22 and 7.23). By what mechanism does the reaction in eq. 8.8 occur? H,sO CH3CHOH + HOCH2CH3 140%. CH,CHOC ethanol diethyl ether
-
Mike has the following monthly information: Salary $4,000 Rent 1,800 700 Car payment Investment Income 200 Meals 900 Groceries 700 Student Loan Payment 400 Other Expenses 600 1. What is Mike's...
-
Buzzsaw Manufacturing incurred $267 200 of manufacturing overhead costs during the past year. However, only $232 000 of overhead was applied to production. At the conclusion of the year, the...
-
On January 1, 2017, a rich citizen of the Town of Ristoni donates a painting valued at $300,000 to be displayed to the public in a government building. Although this painting meets the three criteria...
-
Identify each of the following business combinations as being vertical, horizontal, or conglomerate: a. An inboard marine engine company is acquired by an outboard engine manufacturer. b. A cosmetics...
-
Consider the EER diagram in Figure 4-33. Let's make the following assumptions: There are 12,500,000 customers. These customers have altogether 40,000,000 card accounts. Of these 80 percent are...
-
Particulars Opening stock Purchases Purchases return Sales Sales return Wages Miscellaneous charges Salaries General Expenses: Sundry debtors Sundry Creditors Plant and machinery Buildings Furniture...
-
A soft drink bottling company is interested in controlling its filling operation. Random samples of size 4 are selected and the fill weight is recorded. The table below shows the data for 24 samples....
-
Show the products of these eliminationreactions: CH3 ELOH + NaOCH,CH3 a) "CI Br ELOH + NaOCH,CH; b) "CH,CH
-
Show the products of these elimination reactions and indicate which ismajor: OTs . b) CH,OH + CH,0 + OH ELOH CI E:OH + CH,CH,O c)
-
Material misstatements or omissions in Audit Reports: Auditors are required to report internal control weaknesses to management. The auditors however have no responsibility to report internal control...
-
In the circuit of Fig. 4-51 write two loop equations using I 1 and I 2 . Then find the currents and node voltages. A 3A ( 4 3 V 792 B +1 D w 392 12 C
-
The capacitor in the circuit shown in Fig. 7-37 has initial charge Q 0 = 800 C, with polarity as indicated. If the switch is closed at t = 0, obtain the current and charge, for t > 0. 100 V (+ 10 4 F
-
A gift shop sells 400 boxes of scented candles a year. The ordering cost is \($60\) for scented candles, and holding cost is \($24\) per box per year. What is the economic order size for scented...
-
Kay Vickery is angry with Gene Libby. He is behind schedule developing supporting material for tomorrows capital budget committee meeting. When she approached him about his apparent lackadaisical...
-
Tharpe Painting Company is considering whether to purchase a new spray paint machine that costs \($3,000\) . The machine is expected to save labor, increasing net income by \($450\) per year. The...
-
Use the data from exercise #1 to consider another possibility: They could hire a set of workers at the beginning of the year and build inventory. They could then use the inventory (and some...
-
An investor sells a European call on a share for $4. The stock price is $47 and the strike price is $50. Under what circumstances does the investor make a profit? Under what circumstances will the...
-
Simplify each expression. n+ 5+2 +5n
-
At what approximate positions might the following compounds show IRabsorptions? (c) (b) CH CH (a) CH3CH2CH3 CHCCH2CH3CH2 CHCH2C3CH (f) . (d) (e) " CCH2CH2COCH CH
-
Assume you are carrying out the dehydration of 1-methylcyclohexanol to yield 1-methykyclohexene. How could you use infrared spectroscopy to determine when the reaction is complete?
-
Assume that you are carrying out the base-induced de-hydro bromination of 3-bromo-3-methylpentane (Section 11.7) to yield an alkene. How could you use IR spectroscopy to tell which of two possible...
-
*please calculate irr in excel
-
Which of the following would not be a period cost? Research and development Direct materials Office supplies Advertising costs
-
\ table [ [ Activity Cost Pool,Activity Measure,Total Cost,Total Activity ] , [ Machining , Machine - hours,$ 3 3 0 , 0 0 0 , 1 5 , 0 0 0 MHs ] , [ Machine setups,Number of setups,$ 3 0 0 , 0 0 0 , 5...

Study smarter with the SolutionInn App


