At what approximate positions might the following compounds show IRabsorptions? (c) (b) CH CH (a) CH3CH2CH3 CHCCH2CH3CH2
Question:
At what approximate positions might the following compounds show IRabsorptions?
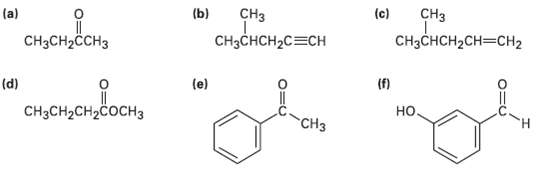
Transcribed Image Text:
(c) (b) CHз CHз (a) CH3CH2CH3 CHзCнCH2CH3CHН2 CHзснCH2C3CH (f) но. (d) (e) "онне CнзCH2CH2COCHз CHз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)
a b c d e Compound CH3CHCCH3 CH32CHCHCCH CH32CHCHCHCH CH3CHCHCOCH3 i CCH3 mol HO O Di...View the full answer

Answered By

Muhammad Ghyas Asif
It is my obligation to present efficient services to my clients by providing a work of quality, unique, competent and relevant. I hope you have confidence in me and assign me the order and i promise to follow all the instructions and keep time.
4.60+
109+ Reviews
203+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
At what approximate positions might the following compounds show IRabsorptions? c??H? " alt="At what approximate positions might the following compounds show IRabsorptions? 51524" class="fr-fic...
-
The following compounds show different rates of debromination. One reacts quite fast, and the other seems not to react at all. Explain this surprising difference in rates. Br KI, acetone Br (CH),C RS...
-
Which of the following compounds show only a single peak in their 1H NMR spectrum? a. CH3CH2OCH2CH3 b. c. CH,CH,CCI
-
Fill in the missing amounts for the following bank reconciliation: Practice Exercise3 Bank Reconciliation March 31,20 Bank Statement Balance $3,764.00 Add: Deposit in transit $4,031.00 Deduct:...
-
Why does Sutton's model apply so well to the consumer goods market? Does Sutton's model describe the structure of other markets?
-
Abercrombie & Fitch sells casual apparel and personal care products for men, women, and children through retail stores located primarily in shopping malls. Its fiscal year ends January 31 of each...
-
The superintendent of the Red Clay School District is concerned about the sick time taken by teachers and administrators. More specifically, she would like to know if there is a difference in the...
-
Martell Mining Companys ore reserves are being depleted, so its sales are falling. Also, its pit is getting deeper each year, so its costs are rising. As a result, the companys earnings and dividends...
-
One-year Treasury securities yield 4.85%. The market anticipates that 1 year from now, 1-year Treasury securities will yield 5.5%. If the pure expectations theory is correct, what is the yield today...
-
Current rhetorical discourse suggests that public sector auditing practices in Ghana are merely symbolic rather than substantive. Some advocates have suggested that in its current state, public...
-
How would you use infrared spectroscopy to distinguish between the following pairs of constitutionalisomers? (a) CH3C=CCH3 and CHCH2C3CH () CHH CHCCH2HH2 and (c) H CH3CH2CHO and
-
Assume you are carrying out the dehydration of 1-methylcyclohexanol to yield 1-methykyclohexene. How could you use infrared spectroscopy to determine when the reaction is complete?
-
Synthesize each of the following compounds by routes that involve benzylic bromination by NBS and any other synthetic steps necessary. Begin by writing a retrosynthetic analysis. (a) (b) (c) CN Br
-
According to the College Board website, the scores on the math part of the SAT (SAT-M) in a certain year had a mean of 507 and a standard deviation of 111. Assume that SAT scores follow a normal...
-
Pay and incentive programs are being used both for knowledge workers and in non-knowledge worker occupations. In every industry, from restaurants to construction and low-tech manufacturing, companies...
-
Closet International invested in an equipment in 2019 with an initial cost of $598,000. It falls under asset class 8 with a CCA rate of 20%. The equipment was sold in 2021 for $260,000. Calculate the...
-
Question 4 (30 Marks) A 12-ply Kevlar/Epoxy composite beam with layup [0/90 / 0 1s is loaded in 3-point bending, as shown in Figure Q4. The beam has a length, L of 100mm, a width, b of 25mm and a...
-
Scenario: You have been working in a community service sector for two years. However, you always find evaluating your own performance challenging. Your Supervisor has also identified that you do not...
-
How does a researcher select the best design?
-
Write a program to move a signed number from smaller register to bigger register. Hint: movzx ax, bl Topic: Data Related Operators and Directives in assembly language
-
An isotonic solution contains 0.90% NaCl mass to volume. Calculate the percent mass to volume for isotonic solutions containing each solute at 25 C. Assume a vant Hoff factor of 1.9 for all ionic...
-
Draw the organic products you would expect to isolate from the following reactions (after hydrolysis). (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) (k) (m) (n) (o) (CH2 = CH)2 CuLi + CH3CH2CH= CHCH2Br...
-
Show how you would use Grignard syntheses to prepare the following alcohols from the indicated starting materials and any other necessary reagents. (a) octan-3-ol from hexanal, CH3 (CH2)4CHO (b)...
-
Give a systematic (IUPAC) name for each diol. (a) CH3CH(OH)(CH2)4 CH(OH)C(CH3)3 (b) HO--(CH2)8-OH (C) (d) (e) HO HO
-
Explain the following: Understand the PPE acquisition (or investing) cycle and related significant transactions and source documents Understand the relevant assertions/objectives about PPE balances...
-
Problem 3 Progress Company acquired 6 0 % of Stall Corporation on 1 2 0 2 0 . Fair values of Stall's assets and liabilities approximated book values on that date. Progress uses the initial value...
-
C: The sor at the poopecin 0ieund to twe oxind places)

Study smarter with the SolutionInn App


