At what approximate positions might the following compounds show IRabsorptions? c??H? alt=At what approximate positions might
Question:
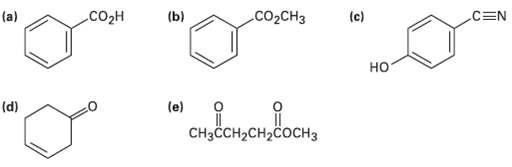
At what approximate positions might the following compounds show IRabsorptions?
 c??H? " alt="At what approximate positions might the following compounds show IRabsorptions? 51524" class="fr-fic fr-dii">
c??H? " alt="At what approximate positions might the following compounds show IRabsorptions? 51524" class="fr-fic fr-dii">
Transcribed Image Text:
(b) со2сHз (c) CEN (a) Соон но "оонен сотн (e) (d) CнзСCH-CH>cосHз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 68% (16 reviews)
Only absorptions OH e ZEU OH with medium to strong intensity are listed aromatic ring CC 145016...View the full answer

Answered By

Stacy kosgei
I offer quality, original and timely services; Highly credible and void of plagiarism. Your success is my pleasure.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
At what approximate positions might the following compounds show IRabsorptions? (c) (b) CH CH (a) CH3CH2CH3 CHCCH2CH3CH2 CHCH2C3CH (f) . (d) (e) " CCH2CH2COCH CH
-
The following compounds show different rates of debromination. One reacts quite fast, and the other seems not to react at all. Explain this surprising difference in rates. Br KI, acetone Br (CH),C RS...
-
Which of the following compounds show only a single peak in their 1H NMR spectrum? a. CH3CH2OCH2CH3 b. c. CH,CH,CCI
-
Following is the chart of accounts of the C. Lucern Clinic: Assets 111 Cash 113 Accounts Receivable 115 Supplies 117 Prepaid Insurance 124 Equipment Liabilities 221 Accounts Payable Owners Equity 311...
-
Zellers and Wal-Mart are two of Canada's largest retailers. To reflect the strong position of the Canadian dollar, each firm is considering lowering prices on some goods in Canadian stores. The table...
-
Exhibit 4.22 presents selected operating data for three retailers for a recent year. Macy??s operates several department store chains selling consumer products such as brand-name clothing, china,...
-
Avon Products has a 24-hour distribution facility in Newark, Delaware, that fulfills orders for the cosmetic makers sales representatives. Orders are filled in one of three distribution lines. Random...
-
Suppose the spot exchange rate for the Canadian dollar is Can $1.19 and the six-month forward rate is Can $1.24. a. Which is worth more, a U.S. dollar or a Canadian dollar? b. Assuming absolute PPP...
-
Franklin Corporation produces a single product. The product is both large and expensive, so few units are produced in any month. The production process requires all material to be brought to the shop...
-
You have an obligation to pay $1,000,000 in 3 years from now, and you would like to make an investment now that will enable you to meet this obligation. This investment will be a portfolio containing...
-
Two infrared spectra are shown. One is the spectrum of cyclohexane, and the other is the spectrum of cyclohexane. Identify them, and explain youranswer. (a) 100 80 60 20 - 1000 4000 3500 3000 2500...
-
How would you use infrared spectroscopy to distinguish between the following pairs of constitutionalisomers? (a) CH3C=CCH3 and CHCH2C3CH () CHH CHCCH2HH2 and (c) H CH3CH2CHO and
-
In general, would you expect a growing, healthy company to report cash provided by or used by its operating, investing, and financing activities? Explain.
-
I need help with discussion posts that respond to 3 of these comments. 2 of them being the first on each picture. RUBRIC: articles to mention Coleman, R., & Banning, S. (2006). Network TV news'...
-
2. Best Use of Scarce Resource DigiCom Corporation produces three sizes of television sets: 12-inch screen, 26-inch screen, and 40-inch screen. Revenue and cost information per unit for each product...
-
Gunther invested $15,000 into a segregated fund with a 65% maturity guarantee 10 years ago. The fund is now maturing and has a current market value of $22,261. Gunther decides to withdraw his...
-
(a) Consider the following financial data (in millions of dollars) for Costello Laboratories over the period of 2014-2018: Year Sales Net income Total assets Common equity 2014 $3,800 $500 $3,900...
-
The Pizza Pie 'N Go sells about 2300 one-topping pizzas each month. The circle graph displays the most requested one-topping pizzas, by percentage, for one month. Most Popular One-Topping Pizzas...
-
What is the difference between a null and an alternative hypothesis?
-
Ex. (17): the vector field F = x i-zj + yz k is defined over the volume of the cuboid given by 0x a,0 y b, 0zc, enclosing the surface S. Evaluate the surface integral ff, F. ds?
-
Magnesium citrate, Mg 3 (C 6 H 5 O 7 ) 2 , belongs to a class of laxatives called hyperosmotics, which cause rapid emptying of the bowel. When a concentrated solution of magnesium citrate is...
-
Give systematic (IUPAC) names for the following diols and phenols. (a) (b) (c) (d) HO HO HO HO OH No OH Cl Br
-
Draw the structures of the following compounds. (Includes both new and old names.) (a) triphenylmethanol (b) 4-(chloromethyl)heptan-3-ol (c) 2-cyclohexen-1-ol (d) 3-cyclopentylhexan-3-ol (e)...
-
Predict which member of each pair has the higher boiling point, and explain the reasons for your predictions. (a) Hexan-1-ol or 3,3-dimethylbutan-1-ol (b) Hexan-2-one or hexan-2-ol (c) Hexan-2-ol or...
-
You borrowed $15,000 for buying a new car from a bank at an interest rate of 12% compounded monthly. This loan will be repaid in 48 equal monthly installments over four years. Immediately after the...
-
Discuss how debt restructuring, settlement, or modification works. Discuss the journal entries for debtor and creditor
-
Could CNL be a viable business? If so, under what conditions and what level of production (and, since production is directly related to production workers, employees)? All information provided for...

Study smarter with the SolutionInn App


