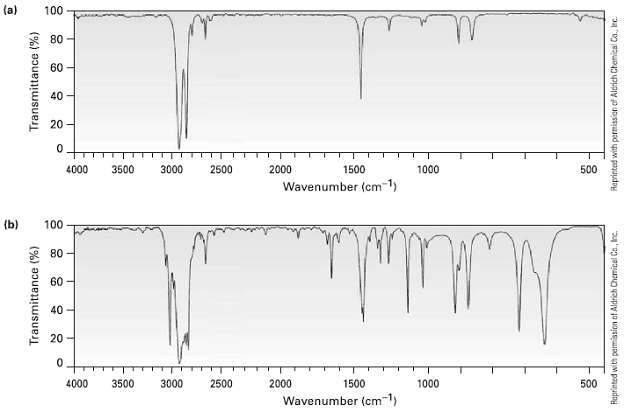
Two infrared spectra are shown. One is the spectrum of cyclohexane, and the other is the spectrum
Question:
Two infrared spectra are shown. One is the spectrum of cyclohexane, and the other is the spectrum of cyclohexane. Identify them, and explain youranswer.
Transcribed Image Text:
(a) 100 80 60 20 - 1000 4000 3500 3000 2500 2000 1500 500 Wavenumber (cm-1) (b) 100 80 60 40 20 - 4000 3500 3000 2500 2000 1500 1000 500 Wavenumber (cm1) Transmittance (%) Transmittance (%) 40 Raprinted with permission of Aldrich Chemicel Co., Inc. Reprinted with permission of Aldrich Chamical Ca. lec.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
Spectrum b differs from spectrum a in several respects Note in part...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Infrared spectra are used by chemists to help identify an unknown substance. Atoms in a molecule that are bound together by a particular bond vibrate at a predictable frequency, and light at that...
-
The infrared spectra for three compounds are provided. Each compound has one or more of the following functional groups: conjugated ketone, ester, amide, nitrile, and alkyne. Determine the functional...
-
Two mass spectra are shown in figure. One spectrum is that of 2-methyl-2-pentene; the other is of 2-hexene. Which is whichexplain? (a) 60 40 20 0. 20 60 80 120 140 10 40 100 m/z (b) 100 80 60 - 10 20...
-
Carey Company is borrowing $200,000 for one year at 12 percent from Second Intrastate Bank. The bank requires a 20 percent compensating balance. What is the effective rate of interest? What would the...
-
Explain why prices are usually strategic complements and capacities are usually strategic substitutes.
-
0.0040 mol of gas undergoes the process shown in FIGURE EX18.35. a. What type of process is this? b. What are the initial and final temperatures in °C? p (atm) 3- 2- V (cm) 300 0+ 100 200 FIGURE...
-
Bagel Boys is a chain that sells New Yorkstyle bagels at three locations. The owner of the chain would like to investigate if there is a difference in the number of bagels sold per day at each...
-
Lowery, Inc. purchased new plant equipment on January 1, 2011. The company paid $920,000 for the equipment, $62,000 for transportation of the equipment, and $10,000 for insurance on the equipment...
-
Sealand Company has 120,000 common shares outstanding. Because it wants to retain its cash for other purposes, the company decided to issue stock dividends to its shareholders. The market price of...
-
Given the activities whose sequence is described by the following table, draw the appropriate activity-on-arrow (AOA) network diagram. (a) Which activities are on the critical path? (b) What is the...
-
How could you use infrared spectroscopy to distinguish between the following pairs of isomers? (a) HC CCH2NH2 and CH3CH2C N (b) CH3COCH3 and CH3CH2CHO
-
At what approximate positions might the following compounds show IRabsorptions? c??H? " alt="At what approximate positions might the following compounds show IRabsorptions? 51524" class="fr-fic...
-
Find the voltage applied to an X-ray tube with nickel anticathode if the wavelength difference between the Ks line and the short-wave cut-off of the continuous X-ray spectrum is equal to 84 pro.
-
The ratio of CEO pay to that of an average employee increased over a period of 50 years from 24:1 to 275:1. Is this increasing gap ethically sound, in your opinion? Should CEO pay be limited in any...
-
Suppose you are considering buying a machine that costs $7,000. It will generate revenues of $1,500 for the next 3 years, and then $1,000 for the following 5 years. What is the payback period of this...
-
National Bakery Limited is the main supplier of a variety of baked products to customers in Kingston. The company currently makes 25,000,000 a variety of baked products annually which uses baking...
-
Q1. Discuss the financial goal of a business. Ensure to provide an example of the inherent ethical challenges associated with the financial goal and or the financial management process. Using the...
-
Q1. How can companies use social media to do sentiment analysis? Describe the process. Give an example of a company that uses sentiment analysis to enhance relationships with customers. Q2. Describe...
-
You are a regional manager for twenty retail stores. Your monthly reports indicate a large number of employees are quitting in five of your stores but very few in the other fifteen stores. What kind...
-
Reduction in sales All of the above 29. Belt of an electric motor is broken, it needs a. Corrective maintenance b. Scheduled maintenance c. Preventive maintenance d. Timely maintenance. 30. The...
-
When HNO 2 is dissolved in water, it partially dissociates according to the equation HNO 2 H + + NO2 . A solution is prepared that contains 7.050 g of HNO 2 in 1.000 kg of water. Its freezing point...
-
For each molecular formula, draw all the possible constitutional isomers of alcohols with that formula. Give the IUPAC name for each alcohol. (a) C3H8O (b) C4H10O (c) C3H6O (d) C3H4O
-
Starting from bromobenzene and any other reagents and solvents you need, show how you would synthesize the following compounds. Any of these products may be used as starting materials in subsequent...
-
Give a systematic (IUPAC) name for each alcohol. Classify each as primary, secondary, or tertiary. (a) (b) (c) (d) (e) (f) (g) Br_CHCH3 OH Cl OH Br OH CI CH2OH
-
Bought an old van for 4000 from Peters promising to pay laterwhat is the transactions
-
Company has a following trade credit policy 1/10 N45. If you can borrow from a bank at 9,5% annual rate, would it be beneficial to borrow money and pay off invoices earlier?
-
Given the following exchange rates, which of the multiple-choice choices represents a potentially profitable inter-market arbitrage opportunity? 129.87/$1.1226/$0.00864/ 114.96/ B $0.8908/ (C)...

Study smarter with the SolutionInn App


