Explain why this carbocation is considerably more stable than this structure would suggest: H +C-0-CH, H
Question:
Explain why this carbocation is considerably more stable than this structure would suggest:
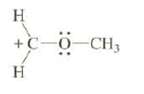
Transcribed Image Text:
H +C-0-CH, H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (15 reviews)
One of the lone pairs of electrons on the oxygen atom of th...View the full answer

Answered By

Sumit kumar
I am an experienced online essay writer with a thorough understanding of any curriculum.and subject expert at Chegg for mathematics, CS subjects..
4.90+
5+ Reviews
13+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Why is isothermal forming considerably more expensive than conventional hot forming?
-
Explain why each of the following alkenes is stable or unstable. (a) 1,2-dimethylcyclopentene (b) trans-1,2-dimethylcyclopentene (c) trans-3,4-dimethylcyclopentene (d) trans-1,2-dimethylcyclodecene...
-
Explain why the enol form of phenol is more stable than the keto form of phenol (eq. 9.43).
-
Kenny operates a store, where he sells feed and other supplies to farmers. Heather purchases a $20,000 tractor from Kenny and pays Kenny with $18,000 in cash and $2,000 in corn. How much gross income...
-
Why might some companies decide not to distinguish the performance of managers from that of their department or division?
-
Your team has been hired to determine the requirements and layout of the Web pages for a company that sells fishing equipment over the Internet. Using the approaches discussed in this chapter,...
-
3 Identify personal qualities which would help a newcomer to quickly adjust to living and working in a foreign country. Give reasons for your choice.
-
The following defined pension data of Doreen Corp. apply to the year 2014. Defined benefit obligation, 1/1/14 (before amendment) .... $560,000 Plan assets, 1/1/14 ................... 546,200 Pension...
-
(Related to Checkpoint 5.7) (Calculating an EAR) Your grandmother asks for your help in choosing a certificate of deposit (CD) from a bank with a one-year maturity and a fixed interest rate. The...
-
Using the alternative-parameter method, determine the parameters of the following distributions based on the given assessments. Refer to Step 5.5 if necessary. a. Find the parameter value for the...
-
Draw the important resonance structures for these species and discuss the contribution of each to the resonance hybrid. Explain whether the species has a large or a small amount of resonance...
-
Explain why one of these anions is much more stable than the other: : a) CH-C-CH-CH b) CH CH3 : CH-C-CH-CH CH-C=N:
-
Anne Teak, the financial manager of a furniture manufacturer, is considering operating a lockbox system. She forecasts that 300 payments a day will be made to lockboxes, with an average payment size...
-
Carrie enjoyed observing wildlife in natural habitats. She wanted to be able to hide at a distance but observe wildlife close up in a variety of circumstances. She visited five different shops and...
-
The operating budget for a certain company shows a net income of $350,000. To achieve this, the company is targeting sales of $637,000, variable costs of $280,280, and fixed costs of $6,720. Compute...
-
What is the output? for (i=0; i
-
a) For silicon, if EG decreases by 0.078 eV, by what fraction does n; increase (assume T is constant at 300K)? b) If the temperature rises from 300K to 600K, by what additional fraction does n;...
-
LOTR (1p) Frodo and Sam need to get to Mordor to destroy the One Ring, but they don't know the exact way. They met a creature called Gollum who claims to know the best way to Mordor. Gollum is...
-
1 How attractive do you think you would find Eden if you worked there, and for what reasons?
-
What is an insurable interest? Why is it important?
-
Find a power series representation for 1/(x + 2).
-
The cholesterol-lowering agents called statins, such as simvastatin (Zocor) and pravastatin (Pravachol), are among the most widely prescribed drugs in the world. Identify the functional groups in...
-
We?ll look at cycloalkanes?saturated cyclic hydrocarbons? and we?ll see that the molecules generally adopt puckered, non-planar conformations. Cyclohexanc, for instance, has a puckered shape like a...
-
We?ll see that there are two isomeric substances both named 1, 2-dimethylcyclohexane. Explain. -C3 1,2-Dimethylcyclohexane CH
-
Fig 1. Rolling a 4 on a D4 A four sided die (D4), shaped like a pyramid (or tetrahedron), has 4 flat surfaces opposite four corner points. A number (1, 2, 3, or 4) appears close to the edge of each...
-
I just need help with question #4 please! Thank you! Windsor Manufacturing uses MRP to schedule its production. Below is the Bill of Material (BOM) for Product A. The quantity needed of the part...
-
(25) Suppose that we have an economy consisting of two farmers, Cornelius and Wheaton, who unsurprisingly farm corn c and wheat w, respectively. Assume that both farmers produce their crop of choice...

Study smarter with the SolutionInn App


