Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate the concentration of I- immediately after the solutions are mixed for trial #1 (answer 0.0300M) Table 1: Volume nf Part 1: Determination of the
Calculate the concentration of I- immediately after the solutions are mixed for trial #1 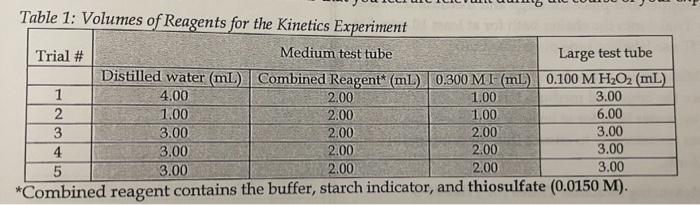


Table 1: Volume nf Part 1: Determination of the Rate Law Procedure 1. Preparation of diluted H2O2 a) Dispense 10.0mL of 1.00MH2O2 into a clean 100 mL volumetric flask and dilute to the mark with distilled water and mix by inversion. Label this solution as diluted H2O2. 2. Preparation of Trials 15 a) Check the condition of your pipet tips and replace any damaged ones. To ensure that you're using the pipet properly, pipet 3.00mL of water into a preweighed beaker and weigh the water. If your water does not weigh 3.00g, ask for help. b) Collect some Combined Reagent in a clean, dry beaker. Record the volume collected and make observations about the solution's appearance. c) Collect some 0.300 M KI in a clean, dry beaker. Record the volume collected and make observations about the solution's appearance. Lab F. Kinetics of Oxidation of lodide Ions b Procedure: d) Pour some diluted HOO into a clean dry beaker and make observations about the solation's appearance. e) Clean and dry 5 medium and 5 large test tubes. Label them Trial 1 to Iirial 5. f) Refer to Table 1 and pipet the required solutions into the medium and large test tubes for Trials 1-5. For Trial 2, pipet 3.00mL of difuted H2O2 twice to get the required 6.00mL 8) Stopper and place the large and medium test tubes for Trial 4 into the shaker bath for at least 10min. Do NOT use labeling tape on this test tube. h) Make an ice water bath in a beaker. Stopper and place the large and medium test tubes for Trial 5 into an ice water bath for at least 10 min. Swirl the test tubes occasionally to ensure that the temperature is uniform. 3. Measuring Reaction Times for Trial \#1-3 a) Quickly pour the contcnts of the medium test tube for Trial 1 into the large test tube for Trial 1. Start timing immediately. Swirl the solution to mix. Record the time (in seconds) it takes for the first appearance of a colour. Repeat this process for Trial 2. b) For Trial 3 , measure and record the temperature of the solution in medium test tube for Trial 3. Repeat the procedure described in 3a ) for Trial 3. 4. Measuring Reaction Times for Trials 45 a) Measure and record the temperature in the medium test tube for Trial 4, then quickly pour the contents of the medium test tube for Trial 4 into the large test tube for Trial 4, Start timing immediately. Return the test tube to the water bath. Swirl the solution to mix. Record the time (in seconds) it takes for the first appearance of a colour. Repeat this process for Trial 5. 5. Clean-up a) Check your results with your instructor before cleaning up. b) Dispose of all colourless solutions down the sink. Dispose of coloured solutions in the fumehood. Wash your glassware with water (answer 0.0300M)



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


