Answered step by step
Verified Expert Solution
Question
1 Approved Answer
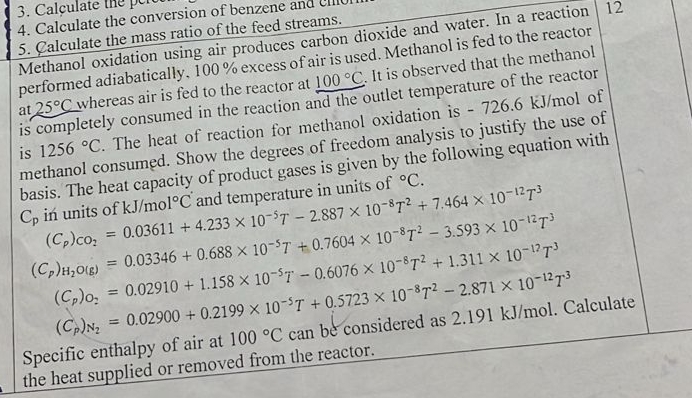
Calculate the conversion of benzene anc Methanol oxidation using air produces carbon dioxide and water. In a reaction performed adiabatically, 1 0 0 % excess
Calculate the conversion of benzene anc
Methanol oxidation using air produces carbon dioxide and water. In a reaction performed adiabatically, excess of air is used. Methanol is fed to the reactor at whereas air is fed to the reactor at It is observed that the methanol is completely consumed in the reaction and the outlet temperature of the reactor is The heat of reaction for methanol oxidation is of methanol consumed. Show the degrees of freedom analysis to justify the use of basis. The heat capacity of product gases is given by the following equation with in units of and temperature in units of
Specific enthalpy of air at can be considered as Calculate the heat supplied or removed from the reactor.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


