Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate the DDT concentration in the spinach sample. Expressthe final answer as milligrams DDT per gram of spinach. A researcher was attemping to quantify the
Calculate the DDT concentration in the spinach sample. Expressthe final answer as milligrams DDT per gram of spinach.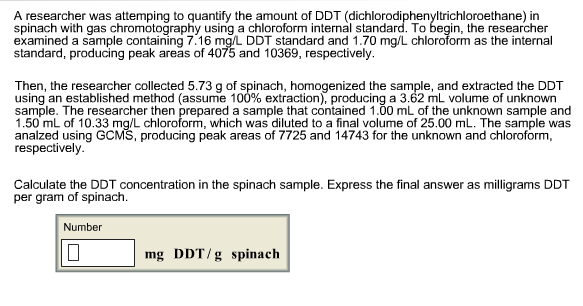
A researcher was attemping to quantify the amount of DDT (dichlorodiphenyltrichloroethane) in spinach with gas chromotography using a chloroform internal standard. To begin, the researcher examined a sample containing 7.16 mg/L DDT standard and 1.70 mg/L chloroform as the internal standard, producing peak areas of 4075 and 10369, respectively. Then, the researcher collected 5.73 g of spinach, homogenized the sample, and extracted the DDT using an established method (assume 100% extraction), producing a 3.62 mL volume of unknown sample. The researcher then prepared a sample that contained 1.00 mL of the unknown sample and 1.50 mL of 10.33 mg/L chloroform, which was diluted to a final volume of 25.00 mL. The sample was analzed using GCMS, producing peak areas of 7725 and 14743 for the unknown and chloroform, respectively. Calculate the DDT concentration in the spinach sample. Express the final answer as milligrams DDT per gram of spinach. Number mg DDT/g spinach
Step by Step Solution
★★★★★
3.33 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1
Internal Standard in Chemical Analysis In performing an analysis an internal standard is a substance ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


