Answered step by step
Verified Expert Solution
Question
1 Approved Answer
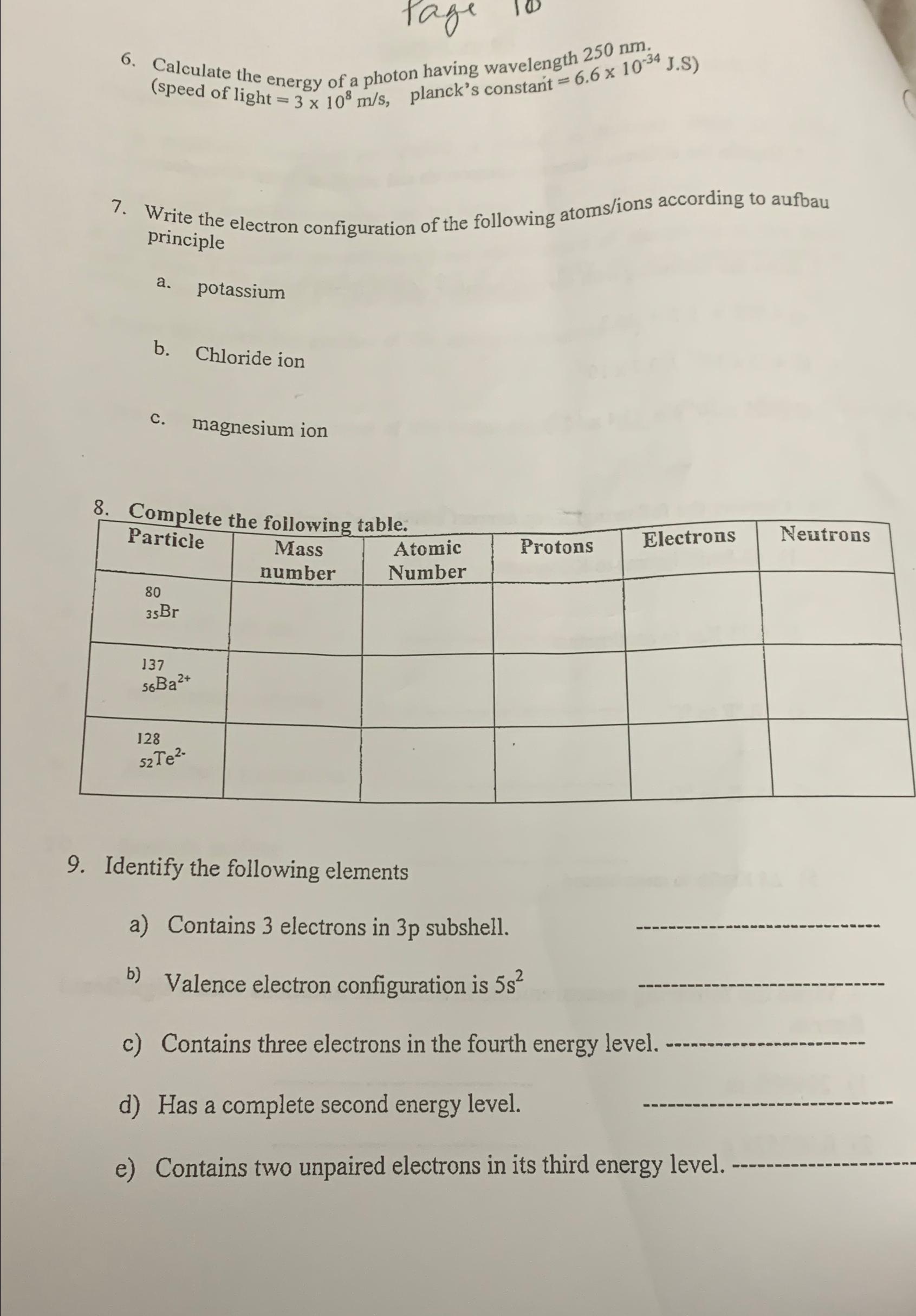
Calculate the energy of a photon having wavelength 2 5 0 n m . ( speed of light = 3 1 0 8 m s
Calculate the energy of a photon having wavelength speed of light planck's constant
Write the electron configuration of the following atomsions according to aufbau principle
a potassium
b Chloride ion
c magnesium ion
Complete the following table.
tableParticletableMassnumbertableAtomicNumberProtons,Electrons,Neutrons
Identify the following elements
a Contains electrons in subshell.
b Valence electron configuration is
c Contains three electrons in the fourth energy leve
d Has a complete second energy level.
e Contains two unpaired electrons in its third energy level.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


