Question
Calculate the equilibrium temperature at 500 bar for the alpha and beta phases of metal A if they are in equilibrium at 817oC and 1
Calculate the equilibrium temperature at 500 bar for the alpha and beta phases of metal A if they are in equilibrium at 817oC and 1 bar. The specific gravities of alpha and beta phases are 18.7 and 19.5, respectively. The vapor pressure data are in the picture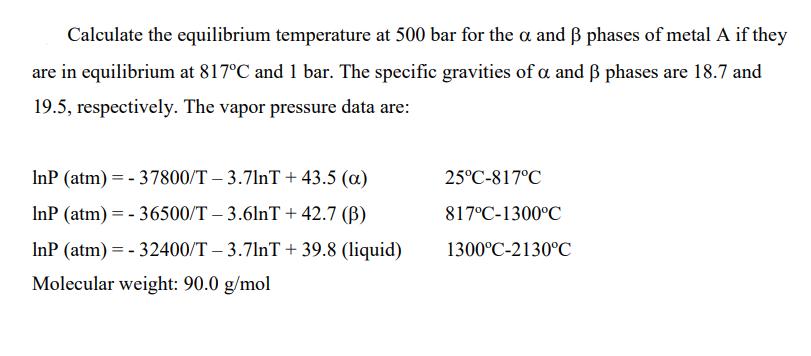
Calculate the equilibrium temperature at 500 bar for the a and B phases of metal A if they are in equilibrium at 817C and 1 bar. The specific gravities of a and phases are 18.7 and 19.5, respectively. The vapor pressure data are: InP (atm) = - 37800/T 3.7lnT + 43.5 (a) 25C-817C InP (atm) = - 36500/T 3.6lnT + 42.7 (B) 817C-1300C InP (atm) = - 32400/T 3.7lnT + 39.8 (liquid) 1300C-2130C Molecular weight: 90.0 g/mol
Step by Step Solution
3.45 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Principles of heat transfer
Authors: Frank Kreith, Raj M. Manglik, Mark S. Bohn
7th Edition
495667706, 978-0495667704
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



