Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate the lattice energy for KBr, which crystalizes in the NaCl lattice using Born Mayer equation (given below) NMZ,ZA (02:-) Useful information radius (K)= 152
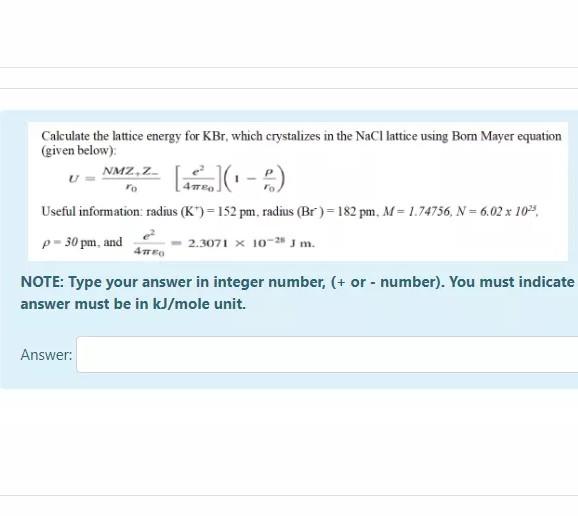
Calculate the lattice energy for KBr, which crystalizes in the NaCl lattice using Born Mayer equation (given below) NMZ,ZA (02:-) Useful information radius (K)= 152 pm, radius (Br)= 182 pm, M=1.74756, N = 6.02 x 10% p - 30pm, and 2.3071 x 10-24 m. 4 NOTE: Type your answer in integer number, (+ or - number). You must indicate answer must be in kJ/mole unit
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


