Question
calculate the molar Flowrates, D1, D2, B1 and B2. Correction: Make Benzene in B2 as 1%. Basis for number 4: _[A]_ (Include the units
calculate the molar Flowrates, D1, D2, B1 and B2.
Correction: Make Benzene in B2 as 1%.
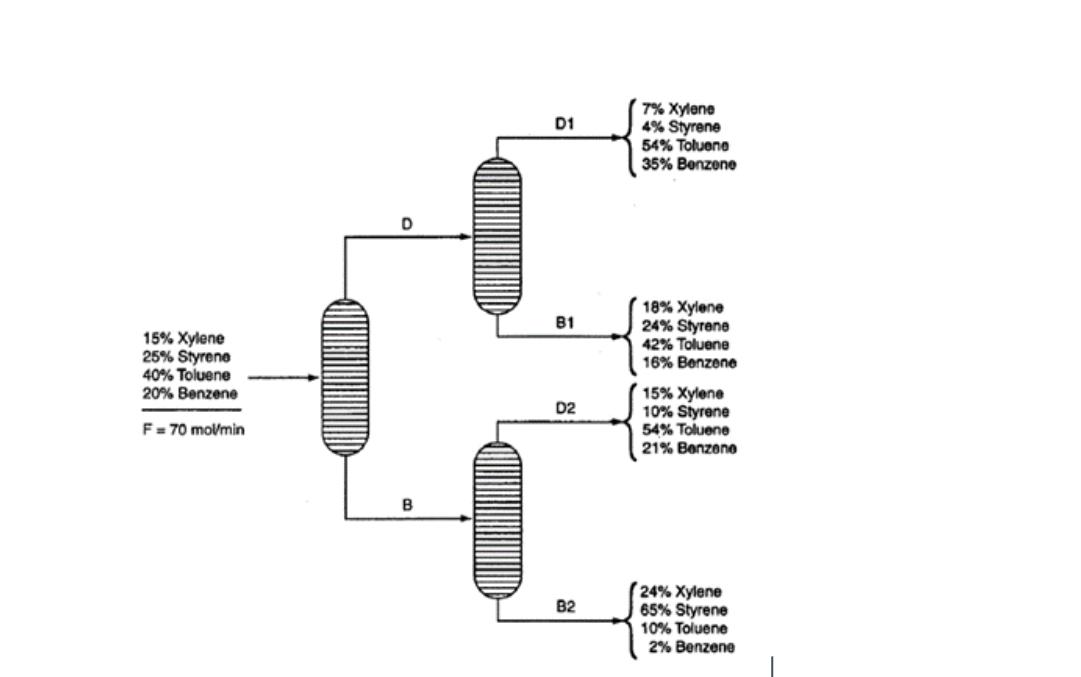
- Basis for number 4: _[A]_
(Include the units in your answer )
- D1 in mol/min
- B1 in mol/min
- D2 in mol/min
- B2 in mol/min
Q5. Fresh Orange juice contains 12.0 wt % solids and the balance water, and concentrate orange juice contains 42 % wt solids. Initially a single evaporation process was used for the concentration, butbvolatile constituents of the juice escaped with the water, leaving the concentrate with a flat taste. The current process overcomes this problem by bypassing the evaporator with a fraction of the fresh juice. The juice that enters the evaporator is concentrated to 58 % wt solids, and the evaporator product stream is mixed with the bypassed fresh juice to achieve the desired final concentration.
a. Draw and label a flowchart of this process, neglecting the vaporization of everything in the juice but water.
b. Calculate the amount of product (42 % concentrate) produced per 100 kg fresh juice fed to the process.
c. Calculate the fraction of the feed that bypasses the evaporator.
For Number 5 letter b:
- The amount of product produced per 100 kg fresh juice fed to the process is ________ kg.
For Number 5 letter c:
- The fraction of feed that bypasses the evaporator is __________.
15% Xylene 25% Styrene 40% Toluene 20% Benzene F = 70 mol/min D B D1 7% Xylene 4% Styrene 54% Toluene 35% Benzene B1 18% Xylene 24% Styrene 42% Toluene 16% Benzene 15% Xylene D2 10% Styrene 54% Toluene 21% Benzene 82 24% Xylene B2 65% Styrene 10% Toluene 2% Benzene
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


