Calculate the molarity of the HCl solution used in the titration in Procedure 8. You should calculate the molarity for each of your three measurements,
Calculate the molarity of the HCl solution used in the titration in Procedure 8. You should calculate the molarity for each of your three measurements, and then calculate the mean molarity (M) and the standard error. You should present you molarity in this form: XXX ± XXX M (± SEM) Show your work. Be sure to consider significant figures in your calculations. The equation for the reaction is:
NaOH + HCl → NaCl + H2O
Hint: You know the concentration of the NaOH (0.01 mole/ L) and you measured the number of mL of this solution used to neutralize the HCl.
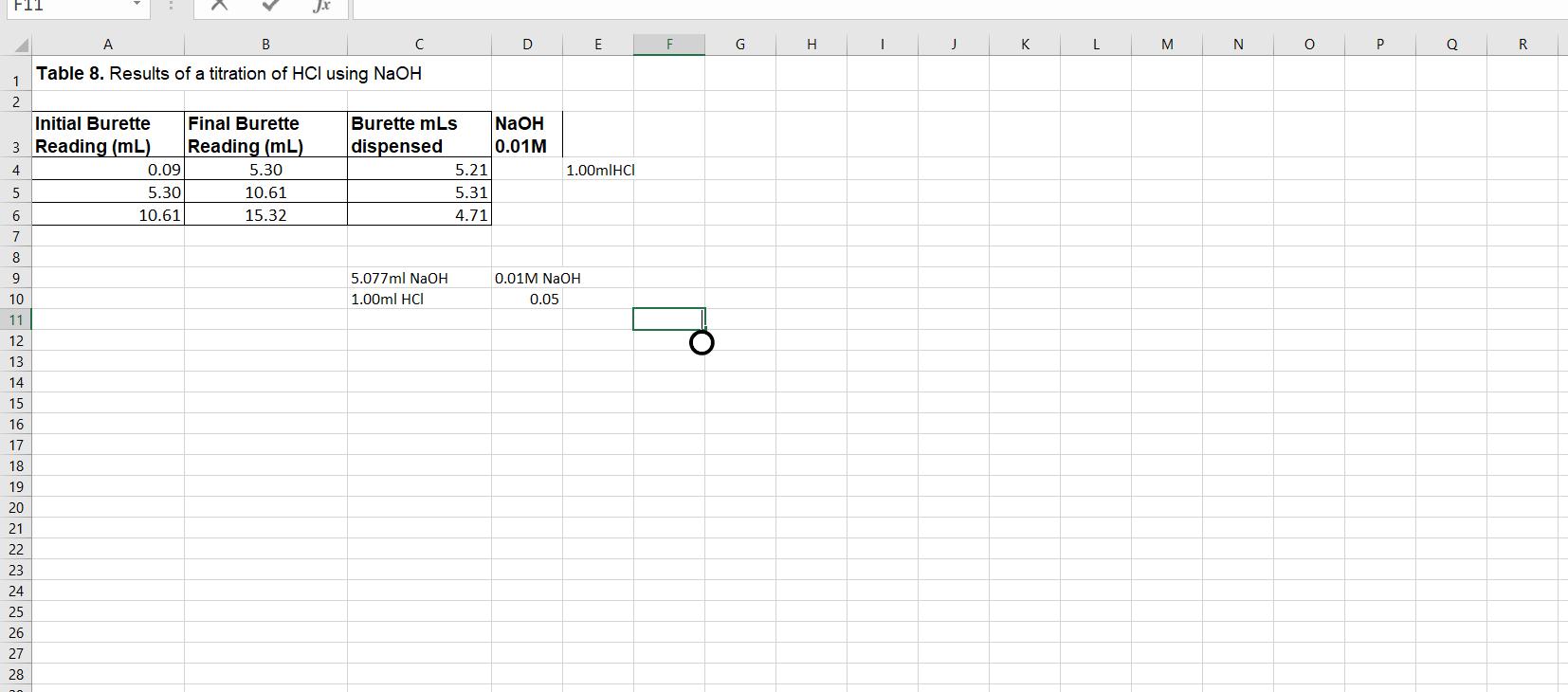
FIT Jx B F G H K L M N P R Table 8. Results of a titration of HCI using NaOH 1 2 Initial Burette Final Burette Burette mLs NaOH 3 Reading (mL) Reading (mL) dispensed 0.01M 4 0.09 5.30 5.21 1.00mlHCI 5 5.30 10.61 5.31 6 10.61 15.32 4.71 7 8 9 5.077ml NaOH 0.01M NaOH. 10 1.00ml HCI 0.05 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28
Step by Step Solution
There are 3 Steps involved in it
Step: 1
After the calculations of the given titration d...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


