Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate the standard enthalpy of reaction (in k(J)/(m)ol ) 2A+B2C+2D where the enthalpies of formation are given in the following table (at 298K ):
Calculate the standard enthalpy of reaction (in
k(J)/(m)ol)\
2A+B2C+2D\ where the enthalpies of formation are given in the following table (at
298K):\ Express your answer in kilojoules per mole (even if the question has kJ only...Instructor\ was unable to fix the Pearson's typo!)\ View Available Hint(s)\
\\\\Delta H_(rxn)\\\\deg = 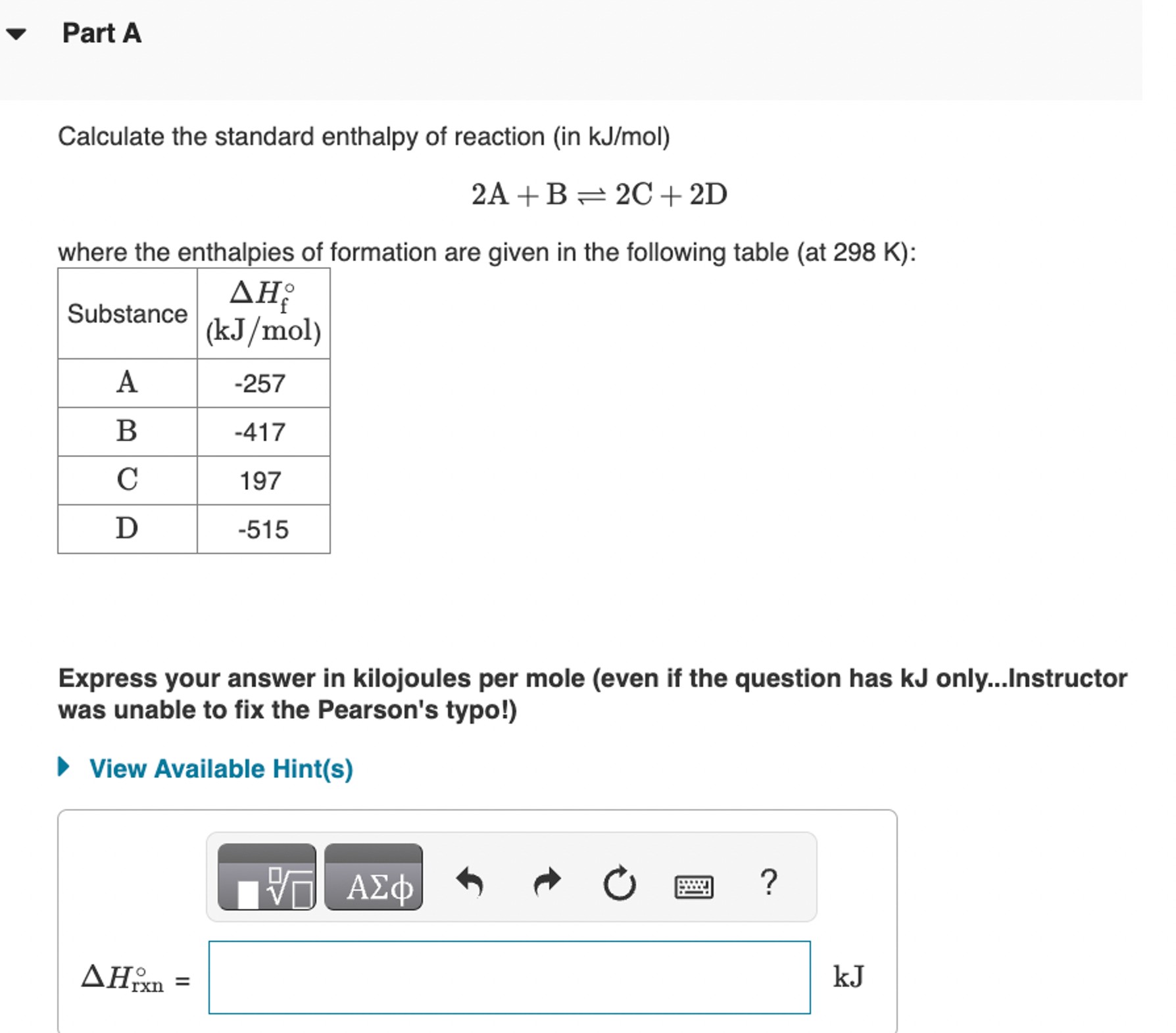
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


