Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate the starting concentration of your unknown acid solution.CExplain why the pH at the equivalence point is not 7 , the pH of neutral water.
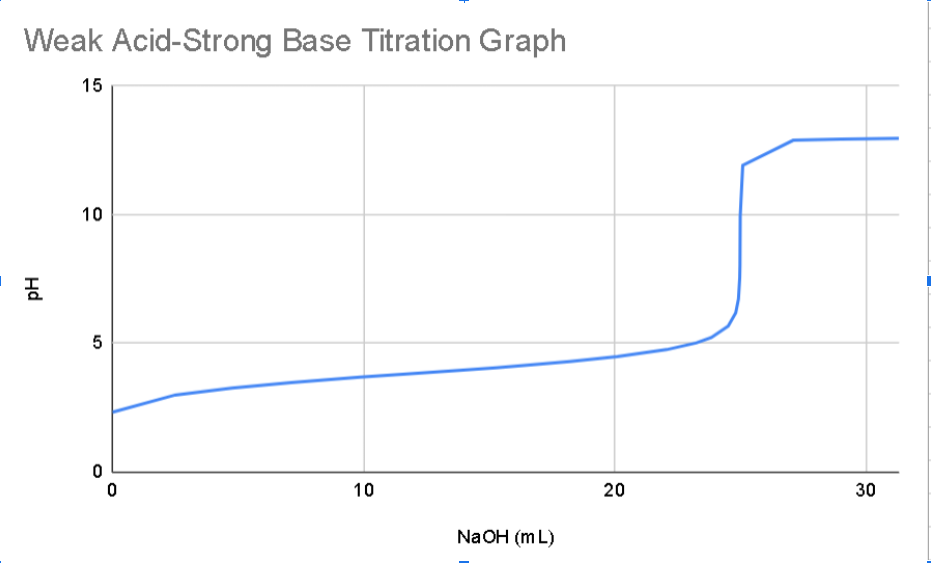
Calculate the starting concentration of your unknown acid solution.CExplain why the pH at the equivalence point is not the pH of neutral water. From your graph, determine the pH and volume of base added at the equivalence point. Determine the point which should correspond to the pKa of your acid and determine what your Ka should be What ions and molecules are present in the solution at the point when half of the volume of base at the equivalence point has been added? How does this compare to a point with double the volume of base compared to the equivalence point? Identify which acid you believe you have and provide your reasoning. For your identified acid, write the balanced chemical equation for the neutralization reaction you performed. Write the Ka expression for your acid. Calculate the HO concentration of your acid before any base is added assuming a starting concentration of M using an ICE table. Determine the ionization of your acid before any base is added. Determine the mass of acid you would have needed to add to the mL of water in order to create the solution in # Assuming you want to turn the solution from # into an ideal buffer solution, approximately what mass of the nd buffer component would you need to add assume the potassium salt
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


