Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculate the theoretical yield of each reaction: Assuming that you started the reaction with 1.0g of each alcohol, determine the theoretical yield of each reaction
Calculate the theoretical yield of each reaction: Assuming that you started the reaction with 1.0g of each alcohol, determine the theoretical yield of each reaction that made for: a. For the synthesis of benzoic acid b. For the synthesis of acetophenone c. For the synthesis of benzaldehyde 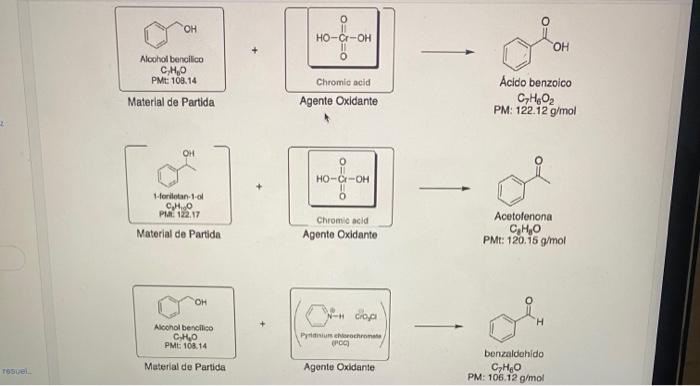
Aloohol bencilico C,H6O PM=108,14 Acido benzoico Material de Partida Agente Oxidante C7H6O2 PM: 122.12g/mol Material de Partida Pit. 122.17 Acetofenona - Chromio acid Agente Oxidante PMt: 120.15g/mol Aconol bereiliso C.HO PMt 10OH.14 Material de Partida benzaldohido C7H6O PM: 106.12g/mol 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


