Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Threonine, an amino acid, has both an acid group and a basic group in its structure (CH3-CH(OH)-CH(NH)-COOH). In aqueous solution it exists predominantly as
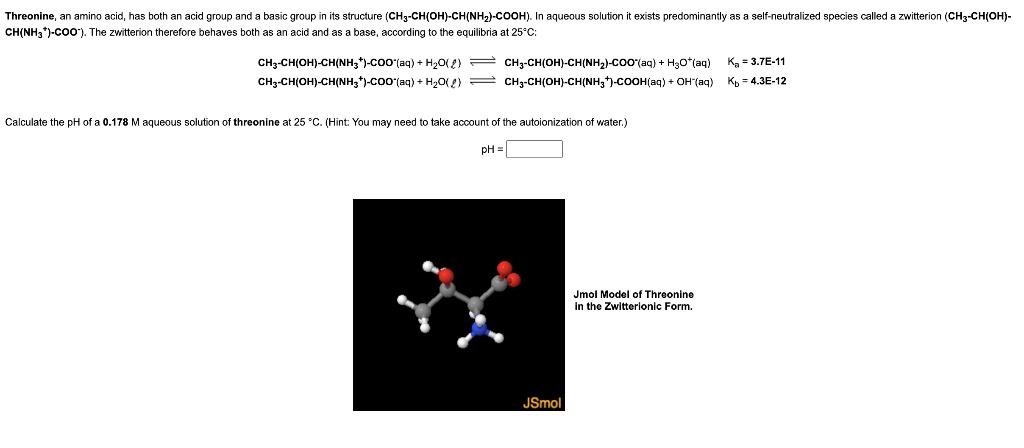
Threonine, an amino acid, has both an acid group and a basic group in its structure (CH3-CH(OH)-CH(NH)-COOH). In aqueous solution it exists predominantly as a self-neutralized species called a zwitterion (CH3-CH(OH)- CH(NH3*)-COO). The zwitterion therefore behaves both as an acid and as a base, according to the equilibria at 25C: CH3-CH(OH)-CH(NH3)-COO(aq) + HO(2) CH3-CH(OH)-CH(NH3)-COO (aq) + HO(2) CH3-CH(OH)-CH(NH,)-COO^(aq) + HyO*(aq) CH3-CH(OH)-CH(NH3)-COOH(aq) + OH (aq) Calculate the pH of a 0.178 M aqueous solution of threonine at 25 C. (Hint: You may need to take account of the autoionization of water.) pH = JSmol Jmol Model of Threonine in the Zwitterionic Form. K = 3.7E-11 Kb =4.3E-12
Step by Step Solution
★★★★★
3.44 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
The problem involves calculating the pH of a solution of the amino acid threonine Threonine has both ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


