Answered step by step
Verified Expert Solution
Question
1 Approved Answer
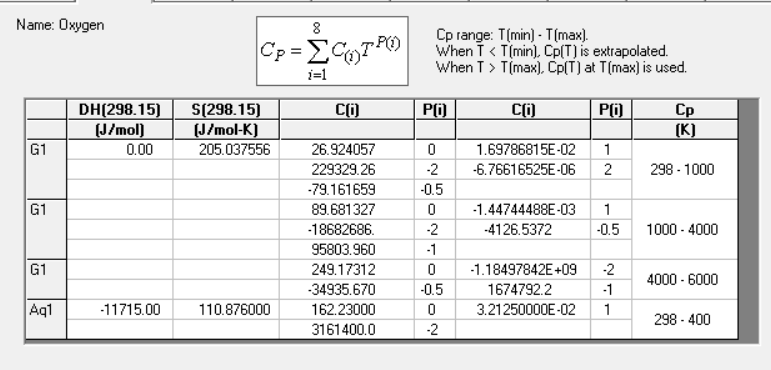
Calculation of Enthalpies and Adiabatic Flame Temperature Ex A fuel gas containing 2 2 % C O , 1 3 % C O 2 and
Calculation of Enthalpies and
Adiabatic Flame Temperature
Ex A fuel gas containing and by volume is combusted with
the theoretically required amount of air in a furnace to heat a solid burden. The gases
enter the furnace at Calculate the adiabatic flame temperature.
Can anyone help me solving this with exel and factsage module?
I know the process without using excel and factsage module. I want to know how to solve with using excel. Also I need accurate anwer. Professor said that he will write this question at his exam. Please help me
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


