Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Calculations for determining the mass percent of Mg2+ in the unknown. Calculations for determining the concentration of Mg2+ at specified points before and after the

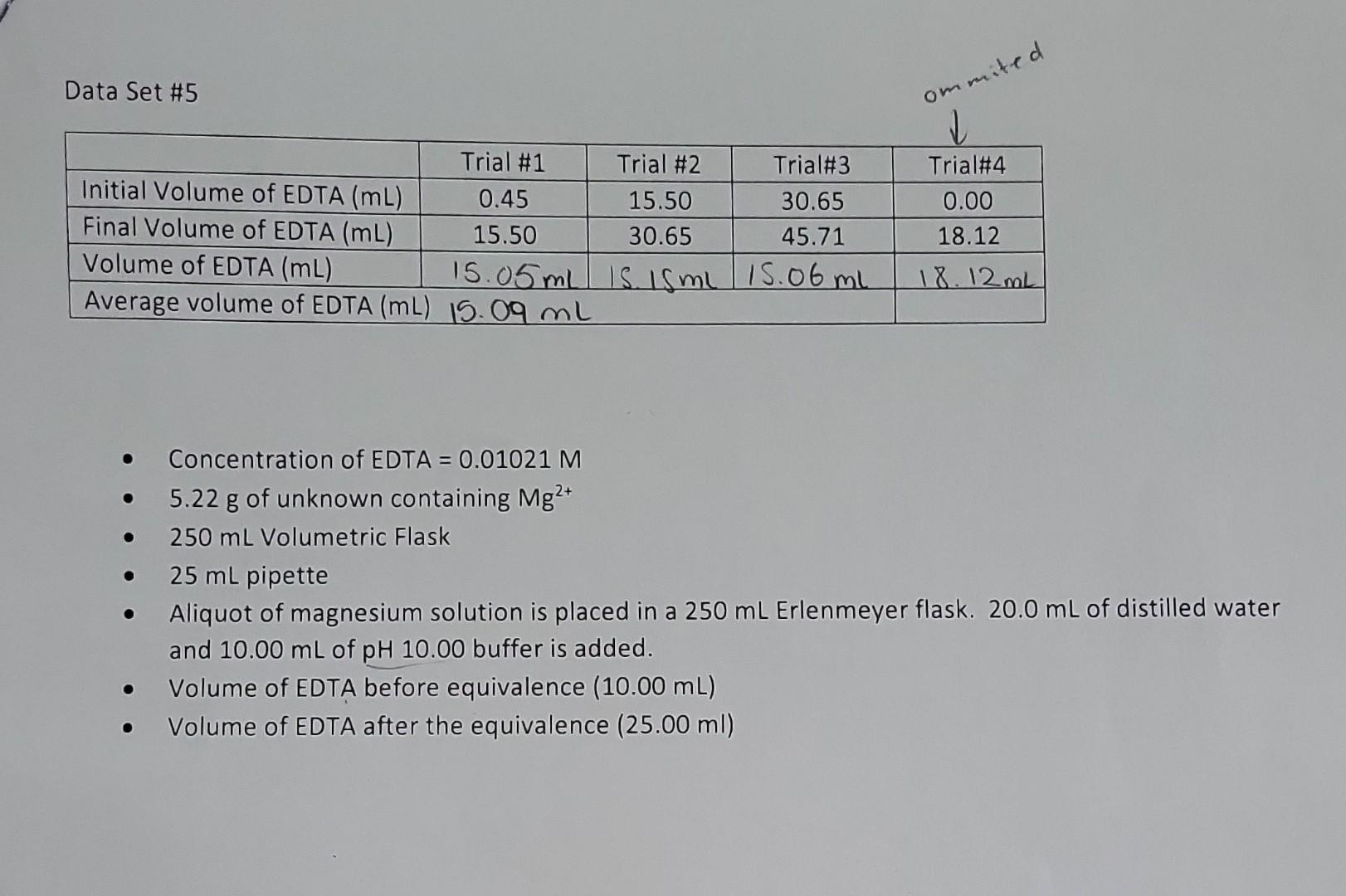
Calculations for determining the mass percent of Mg2+ in the unknown. Calculations for determining the concentration of Mg2+ at specified points before and after the equivalence point. Data Set \#5 - Concentration of EDTA =0.01021M - 5.22g of unknown containing Mg2+ - 250mL Volumetric Flask - 25mL pipette - Aliquot of magnesium solution is placed in a 250mL Erlenmeyer flask. 20.0mL of distilled water and 10.00mL of pH10.00 buffer is added. - Volume of EDTA before equivalence (10.00mL) - Volume of EDTA after the equivalence (25.00ml)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


