Answered step by step
Verified Expert Solution
Question
1 Approved Answer
calulate table (blanks) use the dry lab data to calculate what your experimental R should be. It should be close to the known value of
calulate table (blanks) 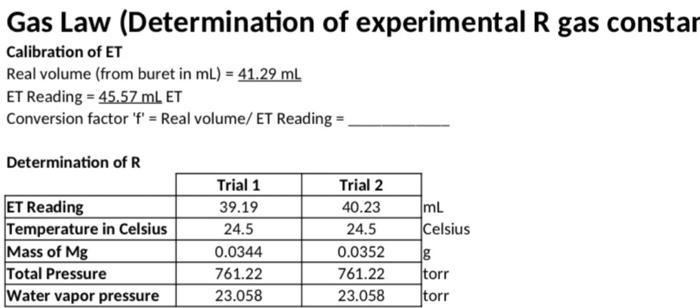
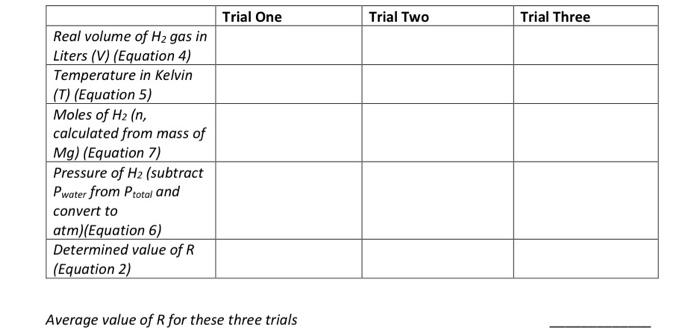
Gas Law (Determination of experimental R gas constar Calibration of ET Real volume (from buret in mL) = 41.29 mL ET Reading = 45.57 mL ET Conversion factor 'f' = Real volume/ ET Reading = Determination of R ET Reading Temperature in Celsius Mass of Mg Total Pressure Water vapor pressure Trial 1 39.19 24.5 0.0344 761.22 23.058 Trial 2 40.23 24.5 0.0352 761.22 23.058 ImL Celsius 8 torr torr Trial One Trial Two Trial Three Real volume of H2 gas in Liters (V) (Equation 4) Temperature in Kelvin (T) (Equation 5) Moles of H2 (n, calculated from mass of Mg) (Equation 7) Pressure of H2 (subtract Pwater from Ptotal and convert to atm)(Equation 6) Determined value of R (Equation 2) Average value of R for these three trials use the dry lab data to calculate what your
experimental R should be. It should be close to
the known value of R that is 0.08206 L atm/mol
K.


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


