Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can anyone help me make this sim on Aspen? thx in advance Chlorobenzene is mainly used as raw material for the synthesis of chemicals including

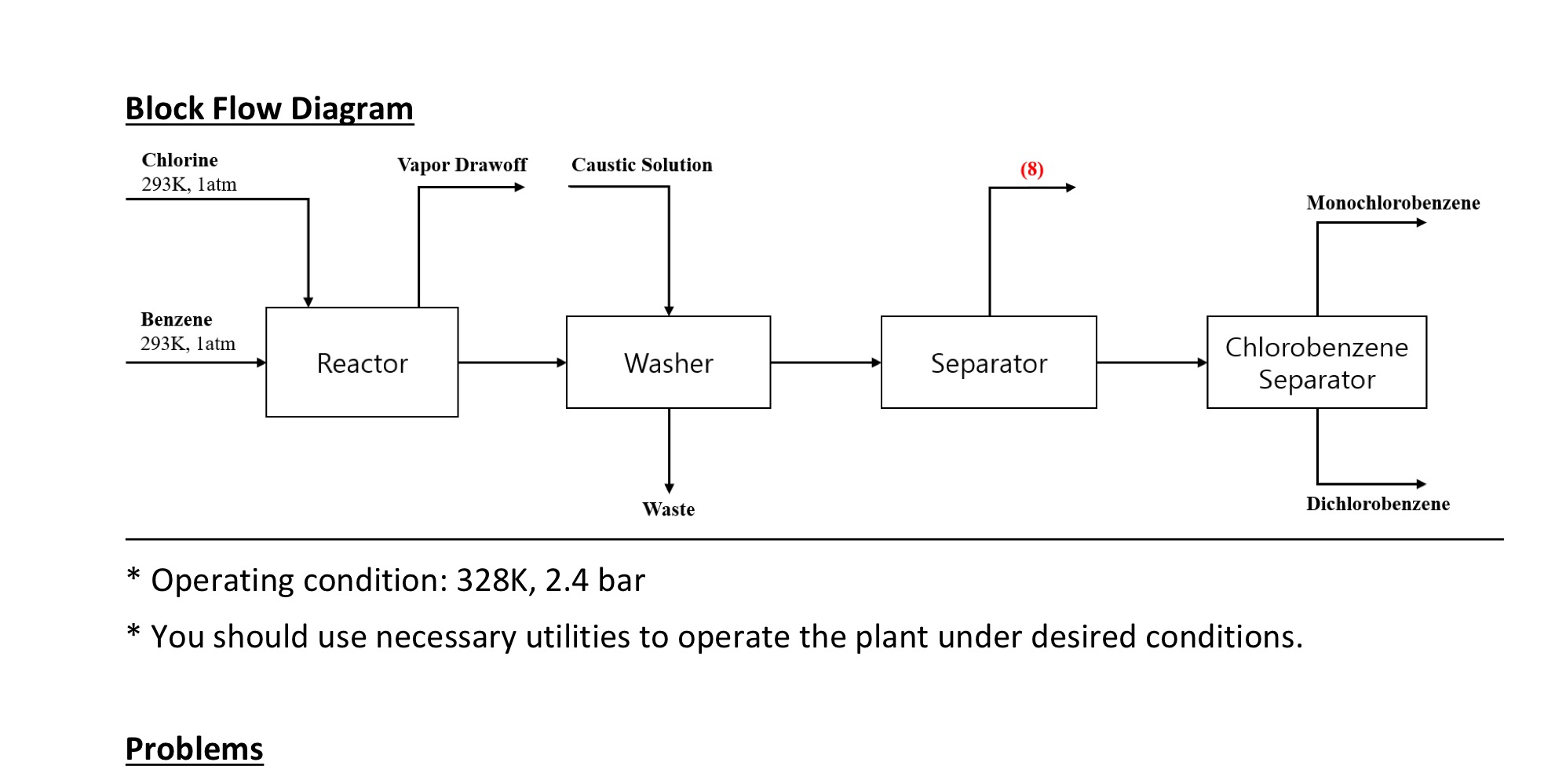
can anyone help me make this sim on Aspen? thx in advance
Chlorobenzene is mainly used as raw material for the synthesis of chemicals including triphenylphosphine, phenylsilane, and thiophenol. These are materials used for organic synthesis, pesticide, and pharmaceutical intermediate. Also, Chlorobenzene was previously used as raw material for the synthesis of o-, p-nitrochlorobenzene and 2,4-dinitrochlorobenzene. In the industry, chlorobenzene is produced by the chlorination of benzene, represented as the chemical equation shown below. As the reaction proceeds, higher substituted chlorobenzene is formed, but in actual industry, only monochlorobenzene and dichlorobenzene is formed under the presence of selective catalysts. C6H6+Cl2C6H5Cl+Cl2C6H5Cl+HClC6H4Cl2+HCl Simulate chlorobenzene production plant with Aspen Plus with the following design specifications. 1) Produce more than 95mol% pure monochlorobenzene and 80 mol\% pure p-dichlorobenzene 2) Feed specification a) Benzene: Flowrate=200kmol/hrPurity=95mol% b) Chlorine gas: Flow rate =500kmol/hr Purity =100mol% Ambient temperature and atmospheric pressure c) Caustic solution: Flow rate =25kmol/hr Composition =10mol%NaOH (balance water) Ambient temperature and atmospheric pressure * Operating condition: 328K,2.4 bar * You should use necessary utilities to operate the plant under desired conditionsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


