Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can anyone help solve this? MISSED THIS? Watch IWE: Obtaining an Empirical Formula from Experimental Data ; Read Section 3.10. You can click on the
Can anyone help solve this? 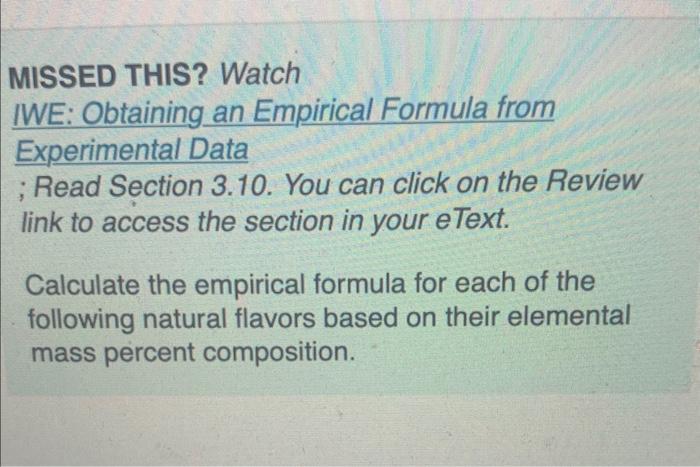
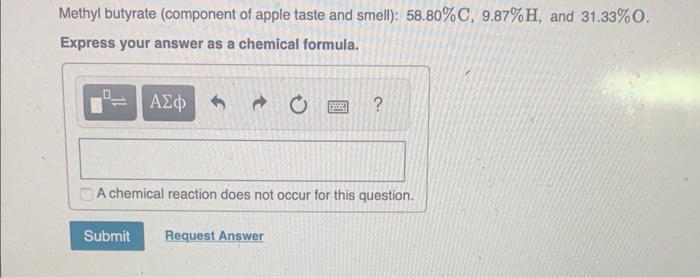
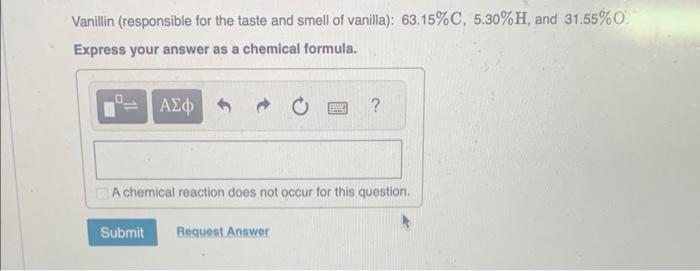
MISSED THIS? Watch IWE: Obtaining an Empirical Formula from Experimental Data ; Read Section 3.10. You can click on the Review link to access the section in your eText. Calculate the empirical formula for each of the following natural flavors based on their elemental mass percent composition. Methyl butyrate (component of apple taste and smell): 58.80%C,9.87%H, and 31.33%O. Express your answer as a chemical formula. Vanillin (responsible for the taste and smell of vanilla): 63.15%C,5.30%H, and 31.55%O. Express your answer as a chemical formula 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


