Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can explain step by step for my quiz? 2. Consider the elementary reaction 2A2B is carried out in a reactor which the catalyst decays by
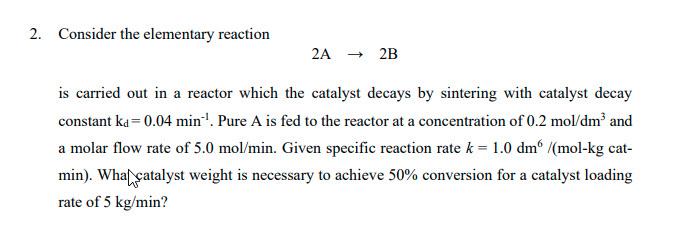
Can explain step by step for my quiz?
2. Consider the elementary reaction 2A2B is carried out in a reactor which the catalyst decays by sintering with catalyst decay constant kd=0.04min1. Pure A is fed to the reactor at a concentration of 0.2mol/dm3 and a molar flow rate of 5.0mol/min. Given specific reaction rate k=1.0dm6/(molkg catmin). Wha catalyst weight is necessary to achieve 50% conversion for a catalyst loading rate of 5kg/minStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


