Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can i get help 1-4. all the information given are at the bottom Questions Important! For all the following questions, your responses must include the
can i get help 1-4. all the information given are at the bottom 

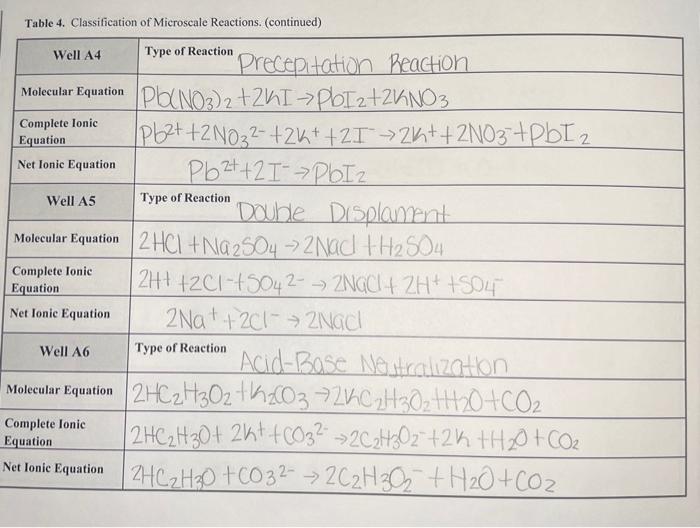
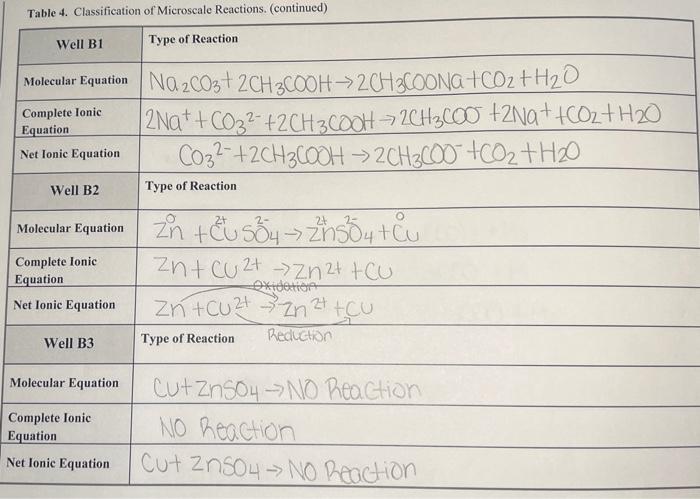
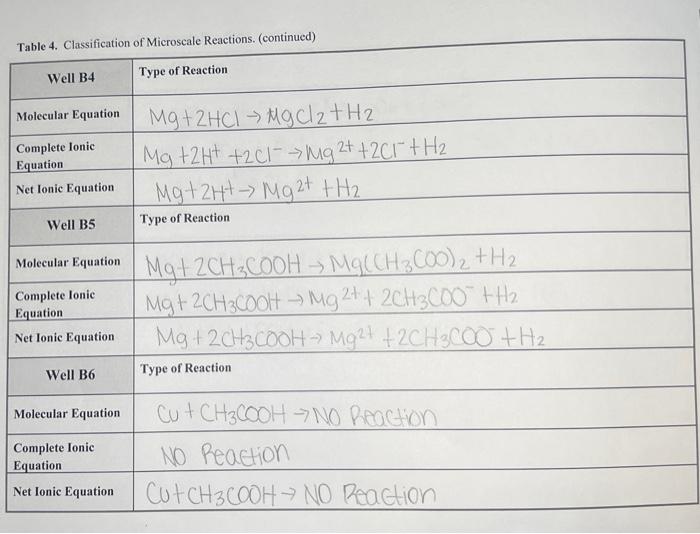

Questions Important! For all the following questions, your responses must include the Well or Test Tube identification and molecular equation" for each example. 1. Does your data illustrate the solubility rules? Provide examples of reactions that formed precipitates. In the molecular equation", circle the precipitate in green. 2. Does your data illustrate metathesis gas-forming reactions? Provide examples, then circle in maize the gas in the "molecular equation". If the gas formed as a result of the decomposition of an unstable intermediate, write the formula for the unstable intermediate in blue under the product side of the "molecular equation". 3. Does your data illustrate the methathesis/formation of weak electrolyte/acid-base reactions? Provide the "molecular equation" and circle the weak electrolyte in green. If a gas was also formed circle the gas in purple. 4. For the reactions categorized as No Reaction, which reaction ClassType were being assessed? Table 4. Classification of Microscale Reactions. Well A1 Type of Reaction Dable displacement Molecular Equation ca(NO3), th2CO3 -> CaCO3+2KNO Complete lonic Equation Ca2+ + 2NO3 +6032 -> CaCO3 +2h++2NO3 Net Ionic Equation Ca2+ +6032 7 Caco 3 Well A2 Type of Reaction 'Double Displacement Molecular Equation AgNO3+ Naci -> Agul + Nano 3 Complete lonie Equation Ag2+ + NO3 + Nat +cf Age + Na+ +NO3 Net Ionic Equation Agt tortAgo! Well A3 Type of Reaction Double Displacement Molecular Equation 2NH4Cl +Na 504 ->(NH4)2504 +2 Nach Complete lonic 2NH4+ + 201"+2Na+ +5042-2NH4+ +504 2- +2Watt 2011 Net lonic Equation NONE Equation Table 4. Classification of Microscale Reactions. (continued) Well A4 Type of Reaction Complete lonic Equation Net Ionic Equation Well A5 Type of Reaction Precepitation Reaction Molecular Equation Pb(NO3)2 +241 ->pbI2+2K NO3 Pb2+ +2 N032- +26+ +21 >2K+ + 2NO3+PBI2 PbZt +21- POIZ Double Displannt Molecular Equation 2 HCI + Na2SO4 -> 2 Nad 1 H2 504 | 2H+ +2C1-+5042- => ZNGCI + 2H+ +504 2 Nat+201 -2NGC Acid-Base Nestualization Molecular Equation 2HC2H302 th 20372KC2H302 44120+CO2 2HC2H30+ 2kt +6032 ->2C2H302 +2k +H20 + CO2 Net lonic Equation 2HC2H30 +6032 -> 2C2H302 + H20+CO2 Complete Ionic Equation Net Ionic Equation Well A6 Type of Reaction Complete Ionic Equation Table 4. Classification of Microscale Reactions. (continued) Well B1 Type of Reaction Complete Ionic Equation Molecular Equation Na2CO3+ 2CH3COOH2CH3COONa+CO2 + H2O | 2Na+ + CO32- +2CH3COOH = 2CH3600 +2Na+ +Coz+ H20 CO32- +2CH36001 > 2CH3600 +oz+H20 Net Ionic Equation Well B2 Type of Reaction Molecular Equation Complete Ionic Equation Net Ionic Equation Zn + EU 554 Zh50 4t cu zn + zn+CU Zt -> Zn2+ tcu xidation Zn +Cu2+ Zn 24 tcu Well B3 Type of Reaction Reduction Molecular Equation Complete Ionic Equation Cut ZnSO4 -> NO Reaction NO Reaction Cut Zn504 - No Reaction Net Ionic Equation Table 4. Classification of Microscale Reactions. (continued) Well B4 Type of Reaction Molecular Equation Complete lonic Equation Net Ionic Equation Mg+2HCl MgCl2 + H2 Mg +2H+ +241--> Mg 2+ +2Cr +H2 Mg + 2H+ Mg 2+ +H2 Well B5 Type of Reaction Molecular Equation Complete Ionic Equation Net Ionic Equation Mgt2CH3COOH MOCCH3COO)2 + H2 Mg+2CH3Cool - Mg 2+4 2CH3C00"+H2. Mg + 2CH3COOH - M924 +2CH3C00+ H2 Well B6 Type of Reaction Molecular Equation Complete Ionic Equation Cut CH3COOH -> NO Picochon NO Reaction Cut CH3COOH NO Reaction Net Ionic Equation Table 4. Classification of Microscale Reactions. (continued) Test Tube 1 Type of Reaction Molecular Equation Complete lonic Equation Net Ionic Equation 2NaOH + H2SO4 Na2504 +2H2O 2Na+ + 20H+2H+ +5042 2Na! +501? + 2H2O + 20H- +2H+ -> 2H20 Test Tube 2 Type of Reaction Molecular Equation Complete Ionic Equation Net Ionic Equation NaOH + CH3COOH -> CH3COONa+ H20 Nat toh- + CH3COOH - CH3COO +Na+ +H2O > HO OH +CH3COOH CH3COO + H20 





Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


