Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can I get help on these two worksheets with work shown! a) Please use EXCEL or another graphing program to plot pH on the yaxis
Can I get help on these two worksheets with work shown! 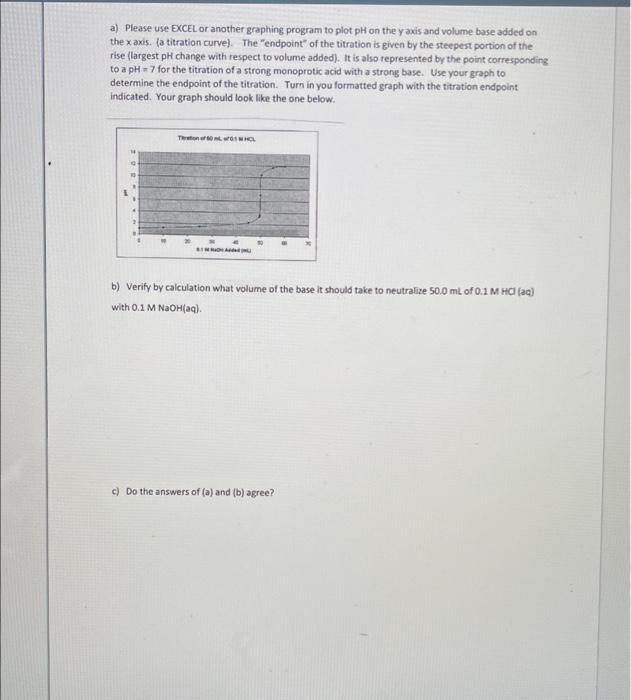
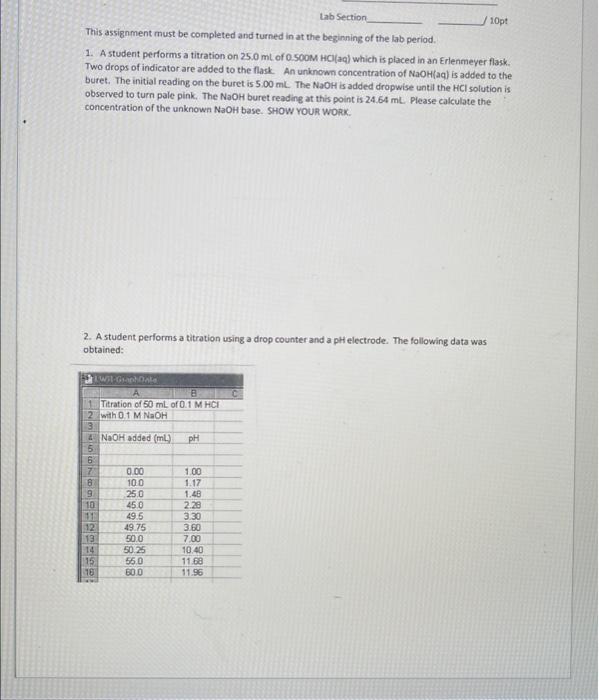
a) Please use EXCEL or another graphing program to plot pH on the yaxis and volume base added on the x axis. (a titration curve). The "endpoint" of the titration is given by the steepest portion of the rise (largest pH change with respect to volume added). It is also represented by the point corresponding to a pH 7 for the titration of a strong monoprotic acid with a strong base. Use your graph to determine the endpoint of the titration. Turn in you formatted graph with the titration endpoint indicated. Your graph should look like the one below. THL b) Verify by calculation what volume of the base it should take to neutralize 50.0 mL of 0.1 M HCI (20) with 0.1 M NaOH(aq). c) Do the answers of (a) and (b) agree? Lab Section 10pt This assignment must be completed and turned in at the beginning of the lab period 1. A student performs a titration on 25.0 ml of 0 SOOM HCl(aq) which is placed in an Erlenmeyer flask. Two drops of indicator are added to the flask An unknown concentration of NaOH(aq) is added to the buret. The initial reading on the buret is 5.00 ml. The NaOH is added dropwise until the HCl solution is observed to turn pale pink. The NaOH buret reading at this point is 24.64 ml. Please calculate the concentration of the unknown NaOH base. SHOW YOUR WORK 2. A student performs a titration using a drop counter and a pH electrode. The following data was obtained: TWIGA 1 Titration of 50 mL of 0.1 MHCE 2 with 0.1 M NaOH NaOH added (ml) pH MOND 10 0.00 100 250 450 49.5 49.75 500 50.25 550 30.0 12 13 14 15 18 1.00 1.17 1.48 2.28 3.30 360 7.00 10.40 11.88 11.96 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


