Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can i have the answers of the 2 brackets inside the circle(actual mass of sa measured and theortical yield lf ASA using h3po4 catalyst) [2
can i have the answers of the 2 brackets inside the circle(actual mass of sa measured and theortical yield lf ASA using h3po4 catalyst) 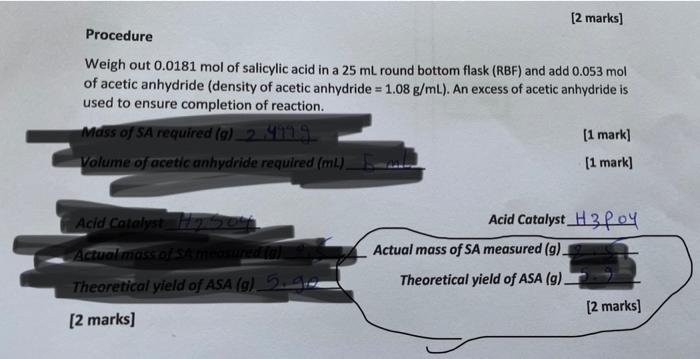
[2 marks] Procedure Weigh out 0.0181mol of salicylic acid in a 25mL round bottom flask (RBF) and add 0.053mol of acetic anhydride (density of acetic anhydride =1.08g/mL ). An excess of acetic anhydride is used to ensure completion of reaction. Mass of SA required (g)2 yil. Volume of acetic anhydride required (mu). [1 mark] [1 mark] Acid Catalyst H P PY Actual mass of SA measured (g) Theoretical yield of ASA(g) [2 marks] 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


