Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can someone compre answers with me? i have answers of my own, just looking to compare them. 1. (2 p) For the following reaction A+2B+2CA+B2C2
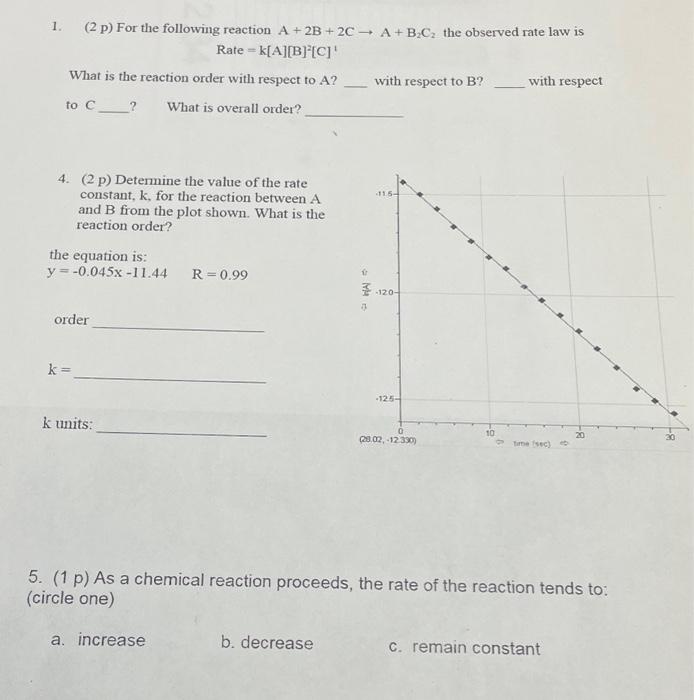
can someone compre answers with me? i have answers of my own, just looking to compare them.
1. (2 p) For the following reaction A+2B+2CA+B2C2 the observed rate law is Rate=k[A][B]2[C]1 What is the reaction order with respect to A ? with respect to B ? with respect to C What is overall order? 4. (2 p) Determine the value of the rate constant, k, for the reaction between A and B from the plot shown. What is the reaction order? the equation is: y=0.045x11.44R=0.99 order k= k units: 5. (1 p) As a chemical reaction proceeds, the rate of the reaction tends to: (circle one) a. increase b. decrease c. remain constant Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


