Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can someone do the materials and methods for the attached data, procedure Procedure Part 1: Determination of analytical wavelength I 1. Record room temperature 2.
can someone do the materials and methods for the attached data, procedure 

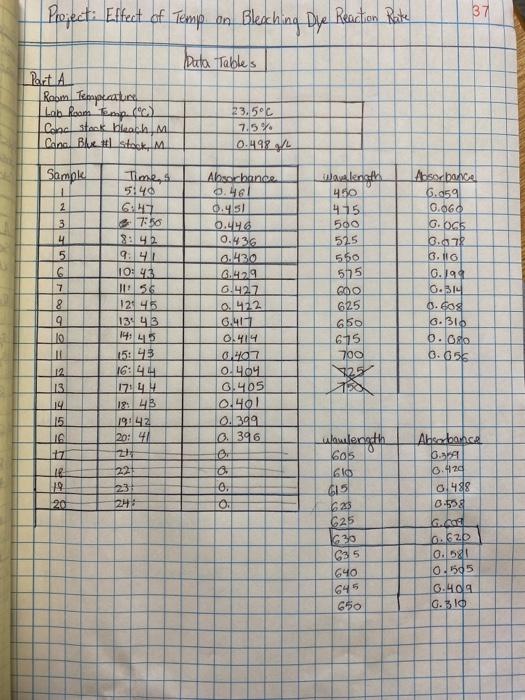

 Procedure Part 1: Determination of analytical wavelength I 1. Record room temperature 2. Record the concentration of the Blue #1 solution and the concentration of the bleach solution? 3. Prepare a blank. a. Fill cuvette with DI water to the calibration line b. Clean cuvette sides with kimwipe C. Place cuvette in spectrophotometer i. Ensure the cuvette is placed with the clear sides facing forward and backward. Use the marked arrow on cuvette for reference when placing d. Press "OABSI/100%T" to set the blank to 0 absorbance e. Remove blank i. DO NOT discard blank, keep to recalibrate later the spectrophotometer between each measurement. 4. Prepare the solution a. Fill cuvette to calibration line with Blue dye #1 5. Set the spectrophotometer wavelength to 450 nm 6. Measure the absorbance of the sample by placing in spectrophotometer a. Clean cuvette and ensure proper directional placement prior to recording measurement, 7. Increase the wavelength by 25 nm a. Record absorbance of sample in spectrophotometer 8. Repeat steps 5-6 by increasing the wavelength by 25nm each time until 750 nm is reached 9. Determine the wavelength with the highest absorbance 10. Determine the analytical wavelength Part 2 1. place in a clean 100 ml beaker, 25ml of blue dye, then place 1 ml of Bleach and mix the two 2. Set the wavefength to the analytical wavelength determined in Part 1. 3. Use BLANK to set A=0. 4. Record the exact concentration of the bleach from the stock bottle. 5. Simultaneously pour the blue dye and bleach solution into a 50 mL beaker. Start timer. Stir the reaction mixture with a glass stirring rod. 6. After 1 minute, rinse a cuvette with approximately 1 mL of the reaction mixture. Pour the rinse in the discard beaker. Fill the cuvette 3/4 full of the reaction mixture. 7. After 5 minutes, measure the absorbance of the reaction mixture. This will be your first absorbance. 8. Record the exact time of the measurement from the timer and the absorbance measurement from the spectrophotometer. Immediately remove the cuvette from the instrument. 9. DO NOT LEAVE THE CUVETTE IN THE SPECTROPHOTOMETER. DO NOT discard the reaction mixture! Project: Effect of Temp on Bleaching Dye 37 Bleaching Dye Reaction Rate Data Tables Part A Room Temperature Lab Room Empo. Colac stock bleach M Cona Blue Stock M 23.5C 7.54 10.498gh Sample 1 2 Times 5140 16:17 7:50 Absorbonica 6.859 0.060 G. 065 0.872 6.10 G.19 0.314 5 6 7 8 9 10 242 9:41 10:43 256 121.415 13 413 wavelength 410 415 500 515 550 575 GOO 625 650 6.75 700 $25 Absorbance 9.46 0:45 O. 0.436 6.430 6.4.219 10.427 0.422 6.411 O.14 WO 0.404 0.405 0.401 0.399 0.396 OF o 0. 0. 14.45 6.310 6.00 0.656 a 12 13 14 15 IS 17 1 19 15:43 16:44 LZL 19. 4B 1904E 20:41 2 221 Wulength 605 231 120 245 GO 015 6.25 C25 630 C35 Absorbance 6.AT 0.420 6.488 0.59 G. 6.620 0.0 0.505 6.ola G. 10 640 645 650 38 Projects Effect of Temp. on Bleaching Dye Reaction Rate Dota Tables (cont.) Part R Temp CIO's increase) Sample 21 Time, s 5:46 700 7 8:03 18:58 199105 INILO 3 55 12:56 13:54 14:55 15:53 | 16:56 17:55 18:52 19:51 20153 86 SWEEP pot Absorbance 6.476 0.463 16.542 G. 2150 O: 446 0440 0.426 0.432 16.429 0424 0.422 0.417 0.414 16.411 6. 40% 0.405 121 17 3 20 1. Materials and Methods - BRIEFLY describe the equipment and procedures (300 words max) o Flow charts, diagrams, pictures o Mention analysis Procedure Part 1: Determination of analytical wavelength I 1. Record room temperature 2. Record the concentration of the Blue #1 solution and the concentration of the bleach solution? 3. Prepare a blank. a. Fill cuvette with DI water to the calibration line b. Clean cuvette sides with kimwipe C. Place cuvette in spectrophotometer i. Ensure the cuvette is placed with the clear sides facing forward and backward. Use the marked arrow on cuvette for reference when placing d. Press "OABSI/100%T" to set the blank to 0 absorbance e. Remove blank i. DO NOT discard blank, keep to recalibrate later the spectrophotometer between each measurement. 4. Prepare the solution a. Fill cuvette to calibration line with Blue dye #1 5. Set the spectrophotometer wavelength to 450 nm 6. Measure the absorbance of the sample by placing in spectrophotometer a. Clean cuvette and ensure proper directional placement prior to recording measurement, 7. Increase the wavelength by 25 nm a. Record absorbance of sample in spectrophotometer 8. Repeat steps 5-6 by increasing the wavelength by 25nm each time until 750 nm is reached 9. Determine the wavelength with the highest absorbance 10. Determine the analytical wavelength Part 2 1. place in a clean 100 ml beaker, 25ml of blue dye, then place 1 ml of Bleach and mix the two 2. Set the wavefength to the analytical wavelength determined in Part 1. 3. Use BLANK to set A=0. 4. Record the exact concentration of the bleach from the stock bottle. 5. Simultaneously pour the blue dye and bleach solution into a 50 mL beaker. Start timer. Stir the reaction mixture with a glass stirring rod. 6. After 1 minute, rinse a cuvette with approximately 1 mL of the reaction mixture. Pour the rinse in the discard beaker. Fill the cuvette 3/4 full of the reaction mixture. 7. After 5 minutes, measure the absorbance of the reaction mixture. This will be your first absorbance. 8. Record the exact time of the measurement from the timer and the absorbance measurement from the spectrophotometer. Immediately remove the cuvette from the instrument. 9. DO NOT LEAVE THE CUVETTE IN THE SPECTROPHOTOMETER. DO NOT discard the reaction mixture! Project: Effect of Temp on Bleaching Dye 37 Bleaching Dye Reaction Rate Data Tables Part A Room Temperature Lab Room Empo. Colac stock bleach M Cona Blue Stock M 23.5C 7.54 10.498gh Sample 1 2 Times 5140 16:17 7:50 Absorbonica 6.859 0.060 G. 065 0.872 6.10 G.19 0.314 5 6 7 8 9 10 242 9:41 10:43 256 121.415 13 413 wavelength 410 415 500 515 550 575 GOO 625 650 6.75 700 $25 Absorbance 9.46 0:45 O. 0.436 6.430 6.4.219 10.427 0.422 6.411 O.14 WO 0.404 0.405 0.401 0.399 0.396 OF o 0. 0. 14.45 6.310 6.00 0.656 a 12 13 14 15 IS 17 1 19 15:43 16:44 LZL 19. 4B 1904E 20:41 2 221 Wulength 605 231 120 245 GO 015 6.25 C25 630 C35 Absorbance 6.AT 0.420 6.488 0.59 G. 6.620 0.0 0.505 6.ola G. 10 640 645 650 38 Projects Effect of Temp. on Bleaching Dye Reaction Rate Dota Tables (cont.) Part R Temp CIO's increase) Sample 21 Time, s 5:46 700 7 8:03 18:58 199105 INILO 3 55 12:56 13:54 14:55 15:53 | 16:56 17:55 18:52 19:51 20153 86 SWEEP pot Absorbance 6.476 0.463 16.542 G. 2150 O: 446 0440 0.426 0.432 16.429 0424 0.422 0.417 0.414 16.411 6. 40% 0.405 121 17 3 20 1. Materials and Methods - BRIEFLY describe the equipment and procedures (300 words max) o Flow charts, diagrams, pictures o Mention analysis
Procedure Part 1: Determination of analytical wavelength I 1. Record room temperature 2. Record the concentration of the Blue #1 solution and the concentration of the bleach solution? 3. Prepare a blank. a. Fill cuvette with DI water to the calibration line b. Clean cuvette sides with kimwipe C. Place cuvette in spectrophotometer i. Ensure the cuvette is placed with the clear sides facing forward and backward. Use the marked arrow on cuvette for reference when placing d. Press "OABSI/100%T" to set the blank to 0 absorbance e. Remove blank i. DO NOT discard blank, keep to recalibrate later the spectrophotometer between each measurement. 4. Prepare the solution a. Fill cuvette to calibration line with Blue dye #1 5. Set the spectrophotometer wavelength to 450 nm 6. Measure the absorbance of the sample by placing in spectrophotometer a. Clean cuvette and ensure proper directional placement prior to recording measurement, 7. Increase the wavelength by 25 nm a. Record absorbance of sample in spectrophotometer 8. Repeat steps 5-6 by increasing the wavelength by 25nm each time until 750 nm is reached 9. Determine the wavelength with the highest absorbance 10. Determine the analytical wavelength Part 2 1. place in a clean 100 ml beaker, 25ml of blue dye, then place 1 ml of Bleach and mix the two 2. Set the wavefength to the analytical wavelength determined in Part 1. 3. Use BLANK to set A=0. 4. Record the exact concentration of the bleach from the stock bottle. 5. Simultaneously pour the blue dye and bleach solution into a 50 mL beaker. Start timer. Stir the reaction mixture with a glass stirring rod. 6. After 1 minute, rinse a cuvette with approximately 1 mL of the reaction mixture. Pour the rinse in the discard beaker. Fill the cuvette 3/4 full of the reaction mixture. 7. After 5 minutes, measure the absorbance of the reaction mixture. This will be your first absorbance. 8. Record the exact time of the measurement from the timer and the absorbance measurement from the spectrophotometer. Immediately remove the cuvette from the instrument. 9. DO NOT LEAVE THE CUVETTE IN THE SPECTROPHOTOMETER. DO NOT discard the reaction mixture! Project: Effect of Temp on Bleaching Dye 37 Bleaching Dye Reaction Rate Data Tables Part A Room Temperature Lab Room Empo. Colac stock bleach M Cona Blue Stock M 23.5C 7.54 10.498gh Sample 1 2 Times 5140 16:17 7:50 Absorbonica 6.859 0.060 G. 065 0.872 6.10 G.19 0.314 5 6 7 8 9 10 242 9:41 10:43 256 121.415 13 413 wavelength 410 415 500 515 550 575 GOO 625 650 6.75 700 $25 Absorbance 9.46 0:45 O. 0.436 6.430 6.4.219 10.427 0.422 6.411 O.14 WO 0.404 0.405 0.401 0.399 0.396 OF o 0. 0. 14.45 6.310 6.00 0.656 a 12 13 14 15 IS 17 1 19 15:43 16:44 LZL 19. 4B 1904E 20:41 2 221 Wulength 605 231 120 245 GO 015 6.25 C25 630 C35 Absorbance 6.AT 0.420 6.488 0.59 G. 6.620 0.0 0.505 6.ola G. 10 640 645 650 38 Projects Effect of Temp. on Bleaching Dye Reaction Rate Dota Tables (cont.) Part R Temp CIO's increase) Sample 21 Time, s 5:46 700 7 8:03 18:58 199105 INILO 3 55 12:56 13:54 14:55 15:53 | 16:56 17:55 18:52 19:51 20153 86 SWEEP pot Absorbance 6.476 0.463 16.542 G. 2150 O: 446 0440 0.426 0.432 16.429 0424 0.422 0.417 0.414 16.411 6. 40% 0.405 121 17 3 20 1. Materials and Methods - BRIEFLY describe the equipment and procedures (300 words max) o Flow charts, diagrams, pictures o Mention analysis Procedure Part 1: Determination of analytical wavelength I 1. Record room temperature 2. Record the concentration of the Blue #1 solution and the concentration of the bleach solution? 3. Prepare a blank. a. Fill cuvette with DI water to the calibration line b. Clean cuvette sides with kimwipe C. Place cuvette in spectrophotometer i. Ensure the cuvette is placed with the clear sides facing forward and backward. Use the marked arrow on cuvette for reference when placing d. Press "OABSI/100%T" to set the blank to 0 absorbance e. Remove blank i. DO NOT discard blank, keep to recalibrate later the spectrophotometer between each measurement. 4. Prepare the solution a. Fill cuvette to calibration line with Blue dye #1 5. Set the spectrophotometer wavelength to 450 nm 6. Measure the absorbance of the sample by placing in spectrophotometer a. Clean cuvette and ensure proper directional placement prior to recording measurement, 7. Increase the wavelength by 25 nm a. Record absorbance of sample in spectrophotometer 8. Repeat steps 5-6 by increasing the wavelength by 25nm each time until 750 nm is reached 9. Determine the wavelength with the highest absorbance 10. Determine the analytical wavelength Part 2 1. place in a clean 100 ml beaker, 25ml of blue dye, then place 1 ml of Bleach and mix the two 2. Set the wavefength to the analytical wavelength determined in Part 1. 3. Use BLANK to set A=0. 4. Record the exact concentration of the bleach from the stock bottle. 5. Simultaneously pour the blue dye and bleach solution into a 50 mL beaker. Start timer. Stir the reaction mixture with a glass stirring rod. 6. After 1 minute, rinse a cuvette with approximately 1 mL of the reaction mixture. Pour the rinse in the discard beaker. Fill the cuvette 3/4 full of the reaction mixture. 7. After 5 minutes, measure the absorbance of the reaction mixture. This will be your first absorbance. 8. Record the exact time of the measurement from the timer and the absorbance measurement from the spectrophotometer. Immediately remove the cuvette from the instrument. 9. DO NOT LEAVE THE CUVETTE IN THE SPECTROPHOTOMETER. DO NOT discard the reaction mixture! Project: Effect of Temp on Bleaching Dye 37 Bleaching Dye Reaction Rate Data Tables Part A Room Temperature Lab Room Empo. Colac stock bleach M Cona Blue Stock M 23.5C 7.54 10.498gh Sample 1 2 Times 5140 16:17 7:50 Absorbonica 6.859 0.060 G. 065 0.872 6.10 G.19 0.314 5 6 7 8 9 10 242 9:41 10:43 256 121.415 13 413 wavelength 410 415 500 515 550 575 GOO 625 650 6.75 700 $25 Absorbance 9.46 0:45 O. 0.436 6.430 6.4.219 10.427 0.422 6.411 O.14 WO 0.404 0.405 0.401 0.399 0.396 OF o 0. 0. 14.45 6.310 6.00 0.656 a 12 13 14 15 IS 17 1 19 15:43 16:44 LZL 19. 4B 1904E 20:41 2 221 Wulength 605 231 120 245 GO 015 6.25 C25 630 C35 Absorbance 6.AT 0.420 6.488 0.59 G. 6.620 0.0 0.505 6.ola G. 10 640 645 650 38 Projects Effect of Temp. on Bleaching Dye Reaction Rate Dota Tables (cont.) Part R Temp CIO's increase) Sample 21 Time, s 5:46 700 7 8:03 18:58 199105 INILO 3 55 12:56 13:54 14:55 15:53 | 16:56 17:55 18:52 19:51 20153 86 SWEEP pot Absorbance 6.476 0.463 16.542 G. 2150 O: 446 0440 0.426 0.432 16.429 0424 0.422 0.417 0.414 16.411 6. 40% 0.405 121 17 3 20 1. Materials and Methods - BRIEFLY describe the equipment and procedures (300 words max) o Flow charts, diagrams, pictures o Mention analysis
can someone do the materials and methods for the attached data, procedure 





Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


