Can someone help me fill out the rest of the tables and answer the questions from each section. Do all or don't answer at all. The instructions are provided below Please help.
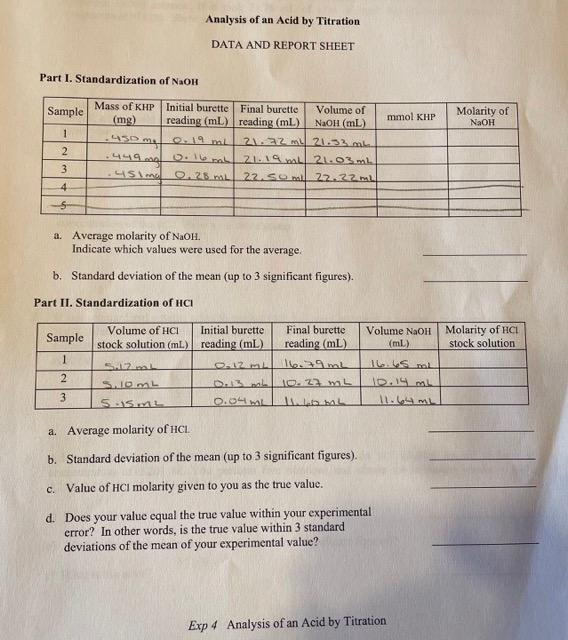




Analysis of an Acid by Titration DATA AND REPORT SHEET Part I. Standardization of NaOH a. Average molarity of NaOH. Indicate which values were used for the average. b. Standard deviation of the mean (up to 3 significant figures). Part II. Standardization of HCl a. Average molarity of HCl. b. Standard deviation of the mean (up to 3 significant figures). c. Value of HCl molarity given to you as the true value. d. Does your value equal the true value within your experimental error? In other words, is the true value within 3 standard deviations of the mean of your experimental value? Exp 4 Analysis of an Acid by Titration 1. Approximately a 0.1MNaOH solution was prepared from analytical grade NaOH pellets, low in NaCO3. The NaOH solution is corrosive and attacks glass. For this reason, this solution should not stand in a burette for extended periods of time. Danger: NaOH is CORROSIVE. Wash off immediately with lots of water if you spill any on yourself. 2. Clamp a burette to a ring stand. Place a 50-mL beaker to collect any droplets from the burette to prevent any contamination. Obtain about 75mL of NaOH in a 100mL beaker. Clean the burette with de-ionized water then with the NaOH solution by filling the burette with each solution and drain it out to the beaker. The waste should go into the waste bucket. 3. Fill the burette with the NaOH solution and be sure all air bubbles have been purged from the burette. The tip of your burette needs to be free of air bubbles too. You may refill the NaOH solution. Record the initial burette reading to the nearest 0.01mL on the DATA AND REPORT SHEET. 4. Accurately measure the mass of three samples of KHP, 0.400g to 0.450g each, into three 125mL Erlenmeyer flasks. Record the mass of samples to the nearest mg on the DATA AND REPORT SHEET. 5. Dissolve the KHP in approximately 30mL of de-ionized water. Add 2-3 drops of phenolphthalein indicator to each flask. Titrate each flask with the NaOH solution, with continuous swirling, until the first faint pink appears which disappears with swirling. 6. Continue to titrate dropwise near the endpoint and use single drops at the endpoint. Wash splashes from the side of the flask into the bulk solution. At the endpoint, the faint pink color will be permanent Exp4 Analysis of an Acid by Titration Chemistry 171L Modern Experimental Chemistry even with swirling. Record the final burette reading to the nearest 0.01mL on the DATA AND REPORT SHEET. The recommended amount of KHP is 2 to 2.2mmol and should require 2023mL of 0.1MNaOH. If your KHP solution does not change color even after you titrate with 25mL of the NaOH solution, you need to do the titration again. 7. Repeat the titration two more times. 8. Calculate the molarity of the NaOH solution using the data from each of the titrations. See Calculations below. Ideally, these molarity values should agree within about 1% or better (0.001M). If the variation is more than 3%(0.003M), run a couple of additional titrations. Any error at this point will be carried on to later parts of the experiment. 9. Average all of the morality of the NaOH solution that you consider reliable. Clearly indicate which values were used for the average. Take this average molarity as the molarity of your standard NaOH solution and use this value to calculate the concentration of the HCl solution in Part II. Calculate the standard deviation of mean of the molarity of the NaOH solution. Calculations Calculations are based on the principle that at the endpoint the number of moles of reactant is stoichiometrically equivalent (i.e., related by stoichiometry) to the number of moles of the other reactant. aA+bBcC+dDnB=abnA where nB is the standard notation for the number of moles of substance B. The molarity of the NaOH solution may be calculated from the fact that the reaction between KHP and NaOH occurs in a 1:1 molar ratio. Hence: nNaOH=nKHPnNaOH=FWofKHPMassofKHP=MNaOHVNaOHMNaOH=VNaOH(inL)nNaOH(inmol) For example, if 0.453g of KHP were titrated with 21.67mL of NaOH, the molarity of NaOH would be calculated as follows: Chemistry 171L Modern Experimental Chemistry MNaOH=VNaOH(inL)nNaOH(inmol)=21.67mL/(1000mL/L)0.002218mol=0.102mol/L=0.102M The mass of the KHP has 3 significant figures while other values have more than 3 significant figures so that it limits the answer to 3 significant figures. Use the data from each of the titrations to calculate the molarity of the NaOH solutions. Pay attention to the proper number of significant figures on the DATA AND REPORT SHEET. Ideally, these duplicates should agree within about 1% or better (0.001M). If the variation is more than 3%(0.003M), it is advisable to run additional samples. Any error at this point will be carried on to later parts of the experiment. Average all of the results that you consider reliable. Clearly indicate which values were used for the average on the DATA AND REPORT SHEET. Calculate the standard deviation of mean of the molarity of the NaOH solution. Take the average molarity as the molarity of your standard NaOH solution and use this value to calculate the concentration of the HCl solution. Part II. Standardization of HCl 1. Obtain about 30mL of the HCl solution in a 50-mL beaker. Use a volumetric pipet to transfer 5.00 mL portions to each of three 125mL Erlenmeyer flasks. Danger: HCl is CORROSIVE. Wash off immediately with lots of water if you spill any on yourself. 2. Using a 50-mL graduated cylinder, add 45mL of de-ionized water to each flask for a total volume of about 50mL. Add 2-3 drops of phenolphthalein. 3. Titrate with the standardized NaOH. Record the initial and final burette reading to the nearest 0.01 mL on the DATA AND REPORT SHEET. 4. Calculate the molarity of the HCl solution for each sample. Precision should be comparable to that of the standardization of KHP. 5. Calculate and report the average molarity and standard deviation of mean of the molarity of the HCl solution. Compare the average molarity to the true value provided from the stock room (Check the whiteboard or ask your instructor). Calculations As the reaction between HCl and NaOH occurs in a 1:1 molar ratio, the molarity of the HCl solution may be calculated in the same way as before. Calculations As the reaction between HCl and NaOH occurs in a 1:1 molar ratio, the molarity of the HCl solution may be calculated in the same way as before. nHCl=nNaOHnHCl=MHClVHCl=MNaOHVNaOH Exp4 Analysis of an Acid by Titration Chemistry 171L Modern Experimental Chemistry MHCl=VHCl(inL)nHCl If 5.00mL of HCl are titrated with 18.64mL of 0.102MNaOH, the molarity is calculated as follows: mmolHCl=mmolNaOH=MNaOHVNaOH(inmL)=0.102M18.64mL=1.901mmolHClMHCl=VHCl(inL)nHCl(inmol)=VHCl(inmL)nHCl(inmmol)=5.00mL1.901mmol=0.380M











