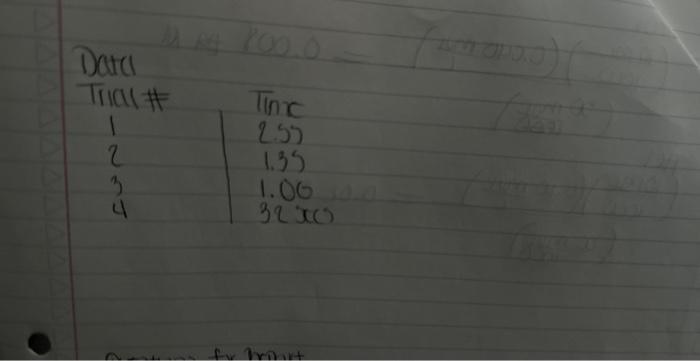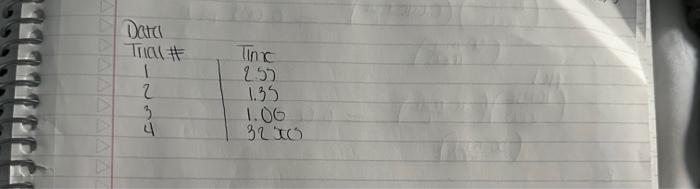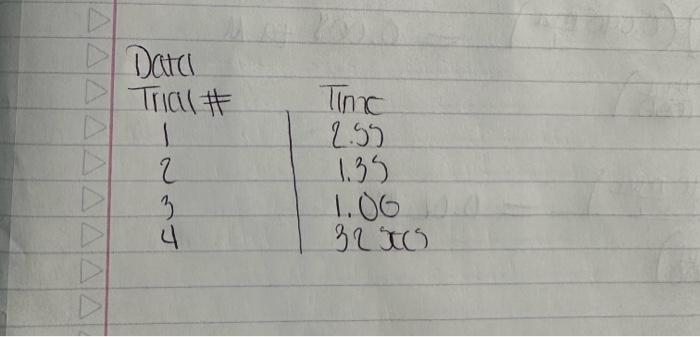Can someone help me solve 2-7
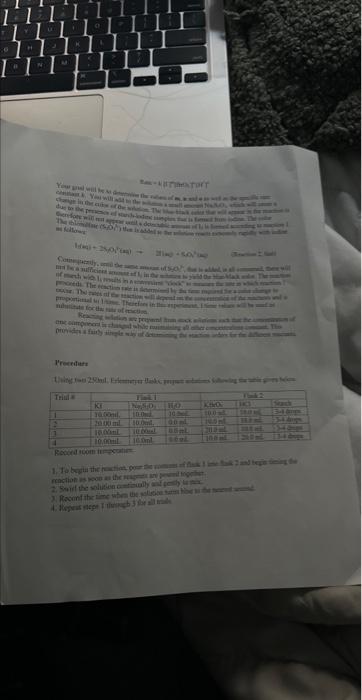
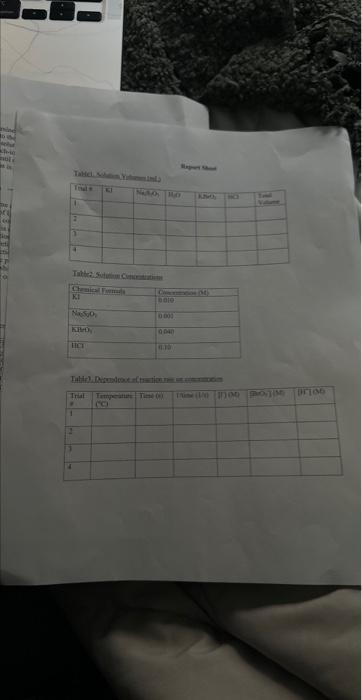
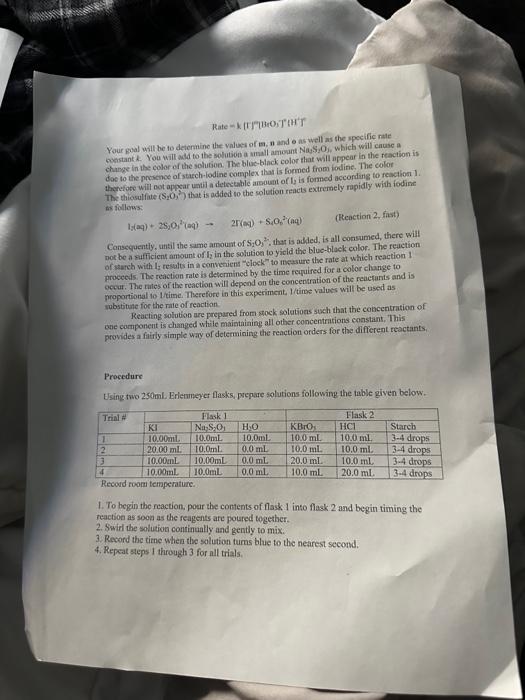
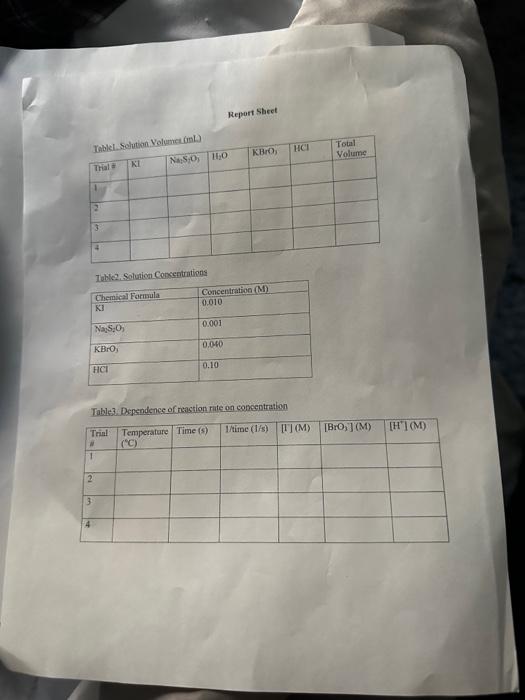
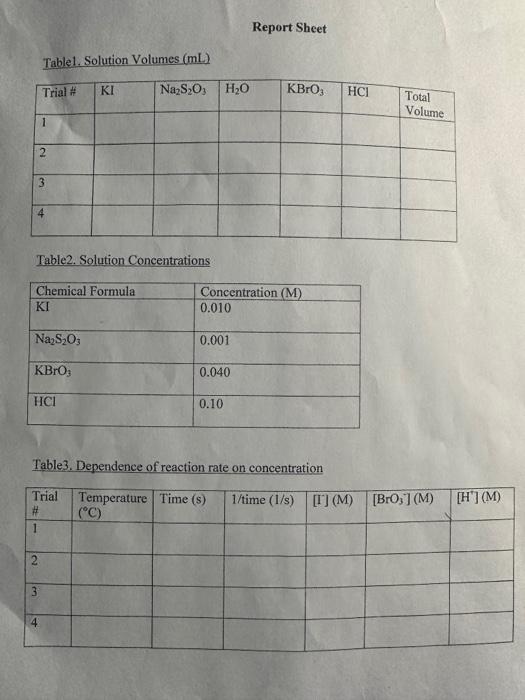
A+Bc+n nis the to te decerind in wi ha tirn man-ipifiarmtart Mrecritura Caly aliatiat Data Trial#12334The2.551.351.00320030 Tntrodectien of varyiny the eoncesiration of reactants. Kinetic is the ares of cheinisfy that deals with the shaty ing of the rbles of reotions. There are foear factors fhat cobtrol roaction filsa in solution. These inclade the sature of the reactanbs, the canceritration of the reactunts, he temperature of the reatice, and the uee of eatalyats. Reactions will oceur when ractand malecule obain emagh cncryy to inferact of oolitite wils other zeacrant ivilecules. Each oee of ihe factors abone aficets tow reactant molecules indenact er collide with eich other. Censider the general resct int schene: A+BC+D The rate of the renction can be ancasaepd by obwerving the rate at wheb the resta n a eeB disapgear of the mate at which products C ar D apiar. Experimentally, the chasye in concentration at A, B. C, and D with time can be measured. The mate of reacteen can be expinesisd ast Rate of disappcarnoe of A Chenge in concentateon of A=B[A] Time nequinet for change Rate of appearaise of C - Chande in scmesteritisn of C - alc Time required for change At The nite law for the general resetion scticene is given as Rake =k[A]2[B], where A amd B are the molar cancentration of reactants, and x and y are orders of the reaction with respect to A and B. k is the rate constant. Adding x and y bogther will give the overall order of the reaction. One nethod of detennining the onder of the reaction is to observe: the changes of the reaction as redetant concentrations are yariod, The stotebiometry and the order of the reaction have no dependence nt each other and usually are ditieren. The mate constant, k, has a definite value for a given reaction and is independent of the conentratioe. Tle rate constan only depends ou tempenature and may be calculsted esce the nufe of the remctiog is knowri. In this experiment, wo will stody the following chemical reactiot: The rate of reaction I depends on the concentration of I.HrOiaudHinwilution.The fate law to be defermined is of the form Your goal will be to deternine the values of an, 3 and o as well as the specific rate change in the color of the solution. The blucblack color that will appear in the reaction is das to the presence of surch-todine corrylex that is formed from iorlinic. The colot therefose will not appear until a detectable amount of ly is formed accordarig to reaction 1. The thiosulfite (S2O32) that is added to the solution reacts exiremely rapidly with fodine is follows: I3(aq)+2S2O12(aq)2F(Aq)+S4Op2(Aq) (Reaction 2 , fist) Cansecqently, until the sume amount of S2O22. that is added, is all consumed, there will not be a rufficient amount of 2 in the solution to yicld the blue-bleck color. The reaction of sarch with la resalts in a conveniens "cloek" to Iteasure the rate at which reaction 1 . proceets. The retiction rate is desermined by the time required for a color change to occur. The riles of the reaction will depend on the concentration of the reactants and is proportional io 1time. Therefore in this expeniment I/time values will be used as mubstitune for the rate of reaction: Keacting wolution are prepared from stock solations such that the cobcentrition of oue cormponent is changed while maiataining all other concentrations canstant, Ihis provides a fairly simple way of deternining the reaction orders for the different reactants, Procedure. Using two 250ml. Eriemmeyer Alasks, psepare solutions following the inble given below. 1. To begin the reaction, poar the contents of flask 1 into flask 2 and begin timing the reactoon as sooa as the reagents afe poured together. 2. Siniri the solution continually and gently to mix. 3. Reword the tirne when the solution furms blue to the nearest second. 4. Repeat steps I through 3 for all trials. Rejort Sheet Table3. Degendence of reastion rite on concentration Colesiatiuns exprestion 3. Wrise and the compeste take las experision Gar reaction 1 . 4. What is the uvidi oruer of the teartiin? 6. Calculat the averaze fate cotrant. (Show yooger veits for the tale conitumt) 7. If yini mint to dotble the rate of the lodine clock waction, what experitseninl fhanges muts to made? \begin{tabular}{l|l} Date \\ Tral\# & Tine \\ 1 & 257 \\ 2 & 1.35 \\ 3 & 1.00 \\ 3 & 3250 \end{tabular} Rates of Chemical Reactions: The Iodine Clock Introduction This experiment will investigate a reaction occurring at different rates as a result of varying the concentration of reactants. Kinetic is the area of chemistry that deals with the studying of the rates of reactions. There are four factors that control reaction rates in solution. These include the nature of the reactants, the concentration of the reactants, the temperature of the reaction, and the use of catalysts. Reactions will occur when reactant molecules obtain enough energy to interact or collide with other reactant molecules. Each one of the factors above affects how reactant molecules interact or collide with each other. Consider the general reaction scheme: A+BC+D The rate of the reaction can be measured by observing the rate at which the reactants A or B disappear or the rate at which products C or D appear. Experimentally, the change in concentration of A,B,C, and D with time can be measured. The rate of reaction can be expressed as: Rate of disappearance of A=TimerequiredforchangeChangeinconcentrationofA=t[A] Rate of appearance of C= Change in concentration of C=[C] Time required for change t The rate law for the general reaction scheme is given as Rate =k[A]x[B]y, where A and B are the molar concentration of reactants, and x and y are orders of the reaction with respect to A and B. k is the rate constant. Adding x and y together will give the overall order of the reaction. One method of determining the order of the reaction is to observe the changes of the reaction as reactant concentrations are varied. The stoichiometry and the order of the reaction have no dependence on each other and usually are different. The rate constant, k, has a definite value for a given reaction and is independent of the concentration. The rate constant only depends on temperature and may be calculated once the rate of the reaction is known. In this experiment, we will study the following chemical reaction: 6Iaq)+BrO3(aq)+6H+(aq)3I2(aq)+Br(aq)+3H2O(Reaction1,slow) The rate of reaction 1 depends on the concentration of I,BrO3 and H+in solution. The rate law to be determined is of the form Your goal will be to determine the values of m,n and o as well as the specific rate constant k. You will add to the solution a small amount Na2S2O3, which will cause a change in the color of the solution. The blue-black color that will appear in the reaction is due to the presence of starch-iodine complex that is formed from iodine. The color therefore will not appear until a detectable amount of I2 is formed according to reaction 1 . The thiosulfate (S2O32) that is added to the solution reacts extremely rapidly with iodine as follows: I2(aq)+2S2O32(aq)2I(aq)+S4O62(aq)(Reaction2,fast) Consequently, until the same amount of S2O32, that is added, is all consumed, there will not be a sufficient amount of I2 in the solution to yield the blue-black color. The reaction of starch with I2 results in a convenient "clock" to measure the rate at which reaction 1 proceeds. The reaction rate is determined by the time required for a color change to occur. The rates of the reaction will depend on the concentration of the reactants and is proportional to 1/ /time. Therefore in this experiment, 1/ time values will be used as substitute for the rate of reaction. Reacting solution are prepared from stock solutions such that the concentration of one component is changed while maintaining all other concentrations constant. This provides a fairly simple way of determining the reaction orders for the different reactants. Procedure Using two 250mL Erlenmeyer flasks, prepare solutions following the table given below. Record room temperature. 1. To begin the reaction, pour the contents of flask 1 into flask 2 and begin timing the reaction as soon as the reagents are poured together. 2. Swirl the solution continually and gently to mix. 3. Record the time when the solution turns blue to the nearest second. 4. Repeat steps 1 through 3 for all trials. Report Sheet Table1. Solution Volumes (mL) Table2. Solution Concentrations Table3. Dependence of reaction rate on concentration Calculations 1. Calculate the exact concentrations of KI,Na2S2O3,KBrO3 and HCl. 2. Using the experimental data collected, evaluate the exponents of the rate law expression. 3. Write out the complete rate law expression for reaction 1. 4. What is the overall order of the reaction? 5. Calculate the rate constant, k, for each trial by substituting the concentration of I, BrO3, and H+and the values of m,n, and 0 into rate law expression. 6. Calculate the average rate constant. (Show proper units for the rate constant) 7. If you want to double the rate of the iodine clock reaction, what experimental changes must be made? Date \begin{tabular}{c|l} Trial\# & Time \\ 1 & 2.55 \\ 2 & 1.39 \\ 2 & 1.00 \\ 3 & 3250) \end{tabular} A+Bc+n nis the to te decerind in wi ha tirn man-ipifiarmtart Mrecritura Caly aliatiat Data Trial#12334The2.551.351.00320030 Tntrodectien of varyiny the eoncesiration of reactants. Kinetic is the ares of cheinisfy that deals with the shaty ing of the rbles of reotions. There are foear factors fhat cobtrol roaction filsa in solution. These inclade the sature of the reactanbs, the canceritration of the reactunts, he temperature of the reatice, and the uee of eatalyats. Reactions will oceur when ractand malecule obain emagh cncryy to inferact of oolitite wils other zeacrant ivilecules. Each oee of ihe factors abone aficets tow reactant molecules indenact er collide with eich other. Censider the general resct int schene: A+BC+D The rate of the renction can be ancasaepd by obwerving the rate at wheb the resta n a eeB disapgear of the mate at which products C ar D apiar. Experimentally, the chasye in concentration at A, B. C, and D with time can be measured. The mate of reacteen can be expinesisd ast Rate of disappcarnoe of A Chenge in concentateon of A=B[A] Time nequinet for change Rate of appearaise of C - Chande in scmesteritisn of C - alc Time required for change At The nite law for the general resetion scticene is given as Rake =k[A]2[B], where A amd B are the molar cancentration of reactants, and x and y are orders of the reaction with respect to A and B. k is the rate constant. Adding x and y bogther will give the overall order of the reaction. One nethod of detennining the onder of the reaction is to observe: the changes of the reaction as redetant concentrations are yariod, The stotebiometry and the order of the reaction have no dependence nt each other and usually are ditieren. The mate constant, k, has a definite value for a given reaction and is independent of the conentratioe. Tle rate constan only depends ou tempenature and may be calculsted esce the nufe of the remctiog is knowri. In this experiment, wo will stody the following chemical reactiot: The rate of reaction I depends on the concentration of I.HrOiaudHinwilution.The fate law to be defermined is of the form Your goal will be to deternine the values of an, 3 and o as well as the specific rate change in the color of the solution. The blucblack color that will appear in the reaction is das to the presence of surch-todine corrylex that is formed from iorlinic. The colot therefose will not appear until a detectable amount of ly is formed accordarig to reaction 1. The thiosulfite (S2O32) that is added to the solution reacts exiremely rapidly with fodine is follows: I3(aq)+2S2O12(aq)2F(Aq)+S4Op2(Aq) (Reaction 2 , fist) Cansecqently, until the sume amount of S2O22. that is added, is all consumed, there will not be a rufficient amount of 2 in the solution to yicld the blue-bleck color. The reaction of sarch with la resalts in a conveniens "cloek" to Iteasure the rate at which reaction 1 . proceets. The retiction rate is desermined by the time required for a color change to occur. The riles of the reaction will depend on the concentration of the reactants and is proportional io 1time. Therefore in this expeniment I/time values will be used as mubstitune for the rate of reaction: Keacting wolution are prepared from stock solations such that the cobcentrition of oue cormponent is changed while maiataining all other concentrations canstant, Ihis provides a fairly simple way of deternining the reaction orders for the different reactants, Procedure. Using two 250ml. Eriemmeyer Alasks, psepare solutions following the inble given below. 1. To begin the reaction, poar the contents of flask 1 into flask 2 and begin timing the reactoon as sooa as the reagents afe poured together. 2. Siniri the solution continually and gently to mix. 3. Reword the tirne when the solution furms blue to the nearest second. 4. Repeat steps I through 3 for all trials. Rejort Sheet Table3. Degendence of reastion rite on concentration Colesiatiuns exprestion 3. Wrise and the compeste take las experision Gar reaction 1 . 4. What is the uvidi oruer of the teartiin? 6. Calculat the averaze fate cotrant. (Show yooger veits for the tale conitumt) 7. If yini mint to dotble the rate of the lodine clock waction, what experitseninl fhanges muts to made? \begin{tabular}{l|l} Date \\ Tral\# & Tine \\ 1 & 257 \\ 2 & 1.35 \\ 3 & 1.00 \\ 3 & 3250 \end{tabular} Rates of Chemical Reactions: The Iodine Clock Introduction This experiment will investigate a reaction occurring at different rates as a result of varying the concentration of reactants. Kinetic is the area of chemistry that deals with the studying of the rates of reactions. There are four factors that control reaction rates in solution. These include the nature of the reactants, the concentration of the reactants, the temperature of the reaction, and the use of catalysts. Reactions will occur when reactant molecules obtain enough energy to interact or collide with other reactant molecules. Each one of the factors above affects how reactant molecules interact or collide with each other. Consider the general reaction scheme: A+BC+D The rate of the reaction can be measured by observing the rate at which the reactants A or B disappear or the rate at which products C or D appear. Experimentally, the change in concentration of A,B,C, and D with time can be measured. The rate of reaction can be expressed as: Rate of disappearance of A=TimerequiredforchangeChangeinconcentrationofA=t[A] Rate of appearance of C= Change in concentration of C=[C] Time required for change t The rate law for the general reaction scheme is given as Rate =k[A]x[B]y, where A and B are the molar concentration of reactants, and x and y are orders of the reaction with respect to A and B. k is the rate constant. Adding x and y together will give the overall order of the reaction. One method of determining the order of the reaction is to observe the changes of the reaction as reactant concentrations are varied. The stoichiometry and the order of the reaction have no dependence on each other and usually are different. The rate constant, k, has a definite value for a given reaction and is independent of the concentration. The rate constant only depends on temperature and may be calculated once the rate of the reaction is known. In this experiment, we will study the following chemical reaction: 6Iaq)+BrO3(aq)+6H+(aq)3I2(aq)+Br(aq)+3H2O(Reaction1,slow) The rate of reaction 1 depends on the concentration of I,BrO3 and H+in solution. The rate law to be determined is of the form Your goal will be to determine the values of m,n and o as well as the specific rate constant k. You will add to the solution a small amount Na2S2O3, which will cause a change in the color of the solution. The blue-black color that will appear in the reaction is due to the presence of starch-iodine complex that is formed from iodine. The color therefore will not appear until a detectable amount of I2 is formed according to reaction 1 . The thiosulfate (S2O32) that is added to the solution reacts extremely rapidly with iodine as follows: I2(aq)+2S2O32(aq)2I(aq)+S4O62(aq)(Reaction2,fast) Consequently, until the same amount of S2O32, that is added, is all consumed, there will not be a sufficient amount of I2 in the solution to yield the blue-black color. The reaction of starch with I2 results in a convenient "clock" to measure the rate at which reaction 1 proceeds. The reaction rate is determined by the time required for a color change to occur. The rates of the reaction will depend on the concentration of the reactants and is proportional to 1/ /time. Therefore in this experiment, 1/ time values will be used as substitute for the rate of reaction. Reacting solution are prepared from stock solutions such that the concentration of one component is changed while maintaining all other concentrations constant. This provides a fairly simple way of determining the reaction orders for the different reactants. Procedure Using two 250mL Erlenmeyer flasks, prepare solutions following the table given below. Record room temperature. 1. To begin the reaction, pour the contents of flask 1 into flask 2 and begin timing the reaction as soon as the reagents are poured together. 2. Swirl the solution continually and gently to mix. 3. Record the time when the solution turns blue to the nearest second. 4. Repeat steps 1 through 3 for all trials. Report Sheet Table1. Solution Volumes (mL) Table2. Solution Concentrations Table3. Dependence of reaction rate on concentration Calculations 1. Calculate the exact concentrations of KI,Na2S2O3,KBrO3 and HCl. 2. Using the experimental data collected, evaluate the exponents of the rate law expression. 3. Write out the complete rate law expression for reaction 1. 4. What is the overall order of the reaction? 5. Calculate the rate constant, k, for each trial by substituting the concentration of I, BrO3, and H+and the values of m,n, and 0 into rate law expression. 6. Calculate the average rate constant. (Show proper units for the rate constant) 7. If you want to double the rate of the iodine clock reaction, what experimental changes must be made? Date \begin{tabular}{c|l} Trial\# & Time \\ 1 & 2.55 \\ 2 & 1.39 \\ 2 & 1.00 \\ 3 & 3250) \end{tabular}