Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can someone help me with molecular Weight? my prof. said my work was wrong Molecular weight of unknown (show calculation) Data - Temperature vs Time
can someone help me with molecular Weight? my prof. said my work was wrong 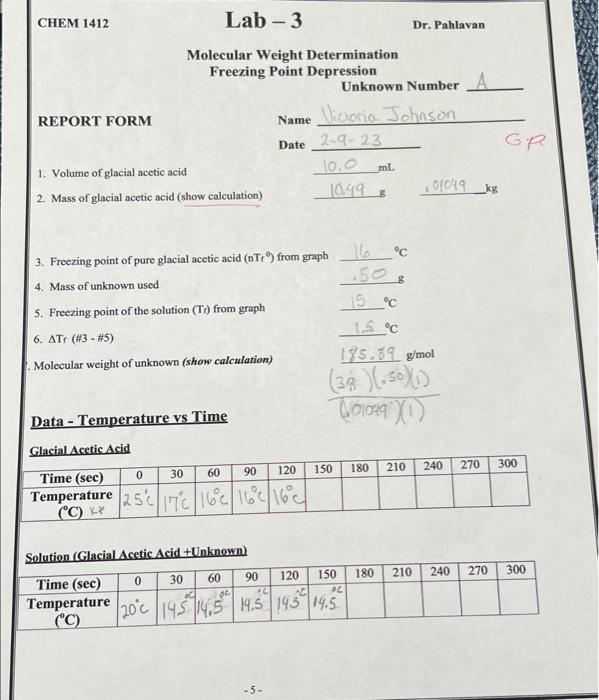

Molecular weight of unknown (show calculation) Data - Temperature vs Time Glacial Acetic Acid Solution (Glacial Acetic Acid + Unknown) CHEM 1412 Lab - 3 MOLECULAR MASS CALCULATIONS The molecular mass (molar mass, MW) of a solute can be determined by changes in the freezing point or boiling point. The freezing point method is more practical because of the greater temperature interval involved. It also can be more accurately measured since the boiling point is more sensitive to atmospheric pressure changes than the freezing point. In this experiment, you will use glacial acetic acid, HC2H3O2, as the solvent. Glacial acetic acid has a freezingpoint of Tf=16.7C and aKf value of 3.9C/m. Since the measured freering point of a substance depends upon its punity, the atmospheric pressure, and the calibration of the thermometer, you must first determine the freezing point of your sample of pure glacial acetic acid under the conditions of this experiment. Then, after adding a measured quantity of an unknown solute to the solvent, you will measure the freezing point of the solution. You can calculate the molecular weight (MW) of the solute from these relationships: 1. Freezing point lowering, Tr=nTr0Tr=Kr.m.i, where nTt0 is the normal freezing point of the pure solvent, Tr is the freezing point of the solution, m is the molality with respect to the solute, and i is the number of solute particles formed in solution (for example, n=2 for NaCl. 2. moles of solute = (grams of solute) /(MW of solute) =g/MW 3. kg of solvent =( grams of solvent )/(1000g/kg) 4. molality of the solution, m= (moles of solute) /(kg of solvent) By substitution, we can derive the following equation for simple determination of molar mass by using freezing point depression: MWsolote=(Tr)(kgwotreat)(Krr)(gsomate)(n)wherenisthenumberofmolesofsoluteparticle(s)permoleofsolute. Example 1 - Calculate the molality of a solution prepared by dissolving 36.0g of glucose, C6H12O6, in 400.0g of water. Solution: Convert grams of glucose to moles of glucose. The molecular mass of glucose is 180.0g. Moles =(36.0g)/(180.0g/mol)=0.200mol glucose The molality expression gives the number of moles of solute (glucose) per one kilogram of solvent (water). Molality =( moles solute /kg solvent )=(0.200mol)/(0.400kg)=0.500m Example 2 - Calculate the freezing point of a solution containing 3.60g of glucose, C6H12O6, in 50.0g of water. The molecular mass of glucose is 180.0g/mol, the normal freezing point of pure water, nTr0, is 0.00C, and Krf for water =1.86C/m. Solution: Tirst calculate the molality of the solution: olality =(3.60g)/(180.0g/mol)(0.0500kg)=0.400m, then calculate Tr: Tf=Kfm.i=(1.86C/m)(0.400m)(1)=0.744C. 2 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


