Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can someone help me with the calculations of the following experiment. I am super lost as I do not know what to do. I appreciate
Can someone help me with the calculations of the following experiment. I am super lost as I do not know what to do.
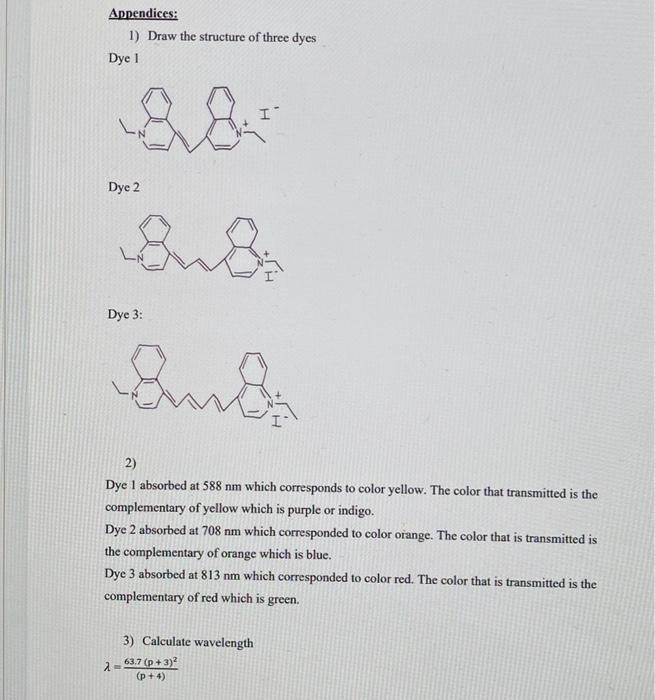
Physical Chemistry Il Lab (CHEM 3132) "Geometry Optimization and Frequency Calculations of three Conjugated Dyes" Background: Computational chemistry, also known as molecular modeling, is a powerful tool to conduct chemical research. It consists of a broad spectrum of techniques applied to predict among other things, molecular geometries, molecular energies, electronic properties, thermodynamic properties, and spectroscopic properties. It is also used to study chemical reactivity, transition states, molecular interactions, and IR, UV, and NMR spectra The equilibrium geometry is defined as the global minimum in a potential energy surface (PES) and it represents the most stable geometry for a chemical species. In this experiment, you will determine the equilibrium geometry from a Geometry Optimization calculation global mamm step localiu Ongle global locali The geometry optimization calculation will be followed by a Frequency Calculation which will compute the harmonic frequencies corresponding to all vibrational modes (3N -6, N = total number of atoms). For an optimized geometry to correspond to the true equilibrium geometry, all harmonic frequency values must correspond to real positive numbers. If any frequency value corresponds to an imaginary number, then the optimized geometry may represent a "saddle point", that is a local minimum in the potential energy surface, instead of the global minimum. If the latter is the case, you will need to resubmit the geometry optimization calculation, The computed IR spectra will be determined from the harmonic frequency values determined during the Frequency Calculation. The theoretical IR spectra will be compared to experimentally determined IR spectra to assess the goodness of your molecular geometries. A ratio of the experimental frequency value to the theoretical frequency value, Vexp/Vtheor 2 0.90 will indicate that your molecular geometries are good representations of the equilibrium geometries of three conjugated dyes. Procedure: You will model all three dyes that you studied in your previous experiment (Exp# 1 Absorption Spectrum of Conjugated Dyes) using Gauss View. For this, you will use the tool Builder found in the VIEW menu. The three conjugated dyes are: Dye 1:1,1'-diethyl-4,4'-cyanine iodide Dye 2: 1,1'-diethyl-4,4'-carbocyanine iodide Dye 3: 1,1'-diethyl-4,4'-dicarbocyanine iodide Week #1 1. You will launch the optimization calculation, which will be followed by a frequency calculations (or you can launch a geometry optimization first, then launch the frequency calculation) using the menu CALCULATE, then selecting the option "Gaussian Calculation Setup". This will allow you to launch a calculation that consists of a geometry optimization which will yield the equilibrium geometry of the chemical specie followed by a frequency calculation that will yield all vibrational modes as well a computed IR spectrum 2. In the "Gaussian Calculation Setup" window you will select the following parameters: Job Type: Opt + Freqor Job Type: Optimization or Frequency Method: Ground State, DFT, Default Spin Basis Sets: 3-21G Charge: 1 (we are running the cation only, no iodide) Spin: Singlet 3. Submit your calculation in order to launch the calculation. You will be prompted to name your input file. 4. Once the calculation is complete. Open the output file generated during the calculation in Gauss View. Using the menu Results and the option Summary, verify that there no imaginary frequencies. In the case there are any imaginary frequencies, the structure needs to be revised and the calculations need to be submitted. 5. Repeat the entire calculation, but using the basis set 6-31G of 3-21G Week # 2 gaurs balance func 6. To access the results of your calculations, use the menu RESULTS (a) Use the option "Summary" for a summary of the results of your calculations. (b) Using the option "Vibrations" get the list of the vibrational modes and their frequencies. (c) Using the option "Vibrations" launch an animation for each vibrational mode using the option "Start Animation". (d) Using the option "Vibrations" followed by the option "Spectrum" obtain the computed IR spectrum. (e) Export the IR spectrum. Data Analysis and Calculations: 1. The HOMO-LUMO energy gap is determined using the "orbital" tool. This energy gap is calculated from the difference in energy between the HOMO and LUMO orbitals. The molecular orbital energies are given in Hartree units. These are some useful conversions: 1 Hartree = 4.35974 x 10-18). 1 Joule = 1 kg m?s 2 Calculate the wavelength in nm (100 m) for each dye using the following hc AE = hva mathematical expression: 2 al=hc 2. Compare the wavelength (max) that you measured in Exp # 1 for each of the three dyes to the calculated wavelengths in this experiment, and determine which basis sets (3-21G or 6-31G) is in better agreement with the experimentally determined wavelength (Imax). 3. Include the computed IR spectra of the 3 dyes in your report. Appendices: 1) Draw the structure of three dyes Dye 1 I Dye 2 La Dye 3: and 2) Dye I absorbed at 588 nm which corresponds to color yellow. The color that transmitted is the complementary of yellow which is purple or indigo. Dye 2 absorbed at 708 nm which corresponded to color orange. The color that is transmitted is the complementary of orange which is blue. Dye 3 absorbed at 813 nm which corresponded to color red. The color that is transmitted is the complementary of red which is green 3) Calculate wavelength 63.7 (P+3) (p+4) 2 Dye 1:1 = 63.7 (1+3)2 (1 + 4) 203.8 nm Dye 2:1 = 63.7 (3 +3)2 327.6 nm (3+4) Dye 3: 2 = 63.7 (5+3)2 = 452.9 nm (5+4) 4) Calculate empirical parameter 2= 63.7 (p+3+ a2 (p + 4) A (p + 4) - (p+3) a = 63.7 Dye 1: a = 588 nm (1+4) 63.7 -(1 + 3) = 2.79 Dye 2: a = 708 nm (3 + 4) (3 + 3) = 2.82 63.7 Dye 3: a = 813 nm(5 + 4) 63.7 -(5 + 3) = 2.72 5) Prepare a table listing the experimental wavelength 2.max and theoretical wavelengths calculated. Experimental wavelength Theoretical wavelength Dye 1 588 nm 204 nm Dye 2 708 nm 328 nm 813 nm 453 nm Dye 3 I appreciate it.
If you need more info, let me know 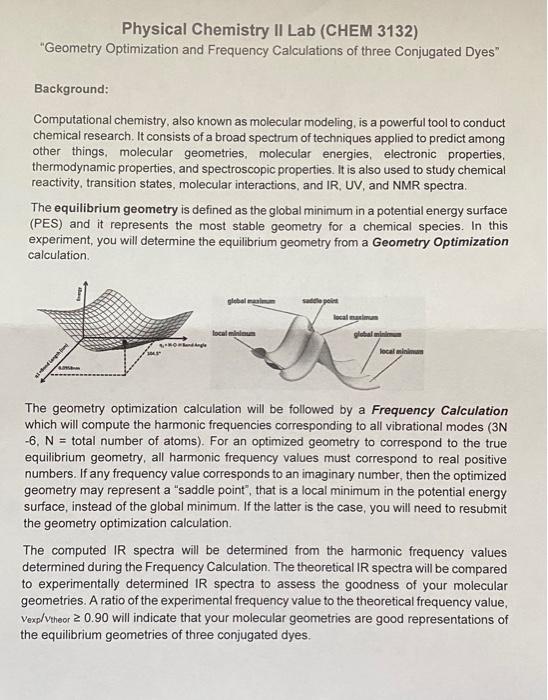








Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


