Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can someone help me with these 3 questions Identify the atomic orbitals in the N-O sigma bond in the following oxime: A. (2sp2,2sp3) B. (2sp,1s)

can someone help me with these 3 questions
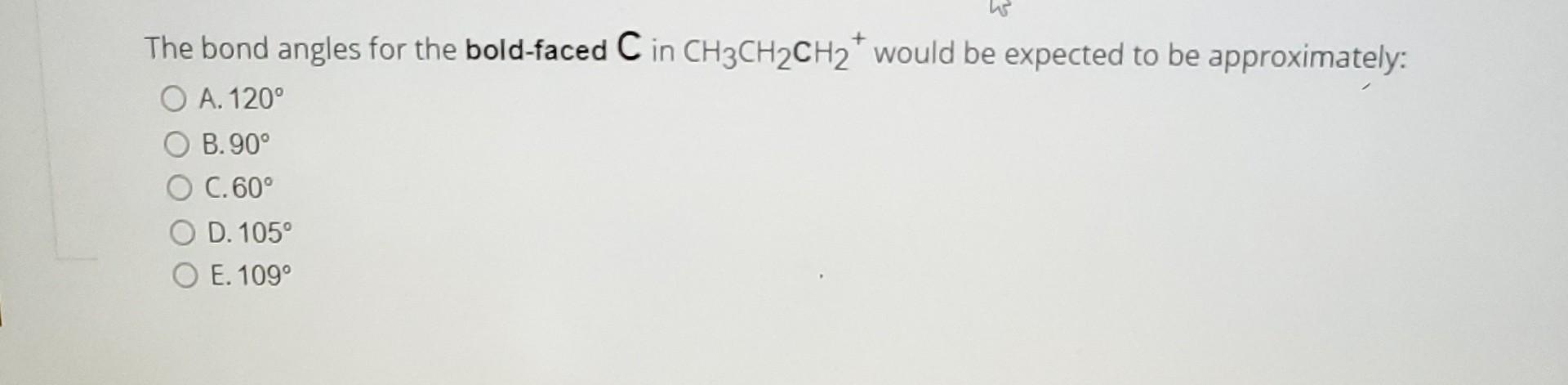

Identify the atomic orbitals in the N-O sigma bond in the following oxime: A. (2sp2,2sp3) B. (2sp,1s) C. (2sp, 2sp) D. (2sp2,2sp2) E. (2sp3,2sp3) The bond angles for the bold-faced C in CH3CH2CH2+would be expected to be approximately: A. 120 B. 90 C. 60 D. 105 E. 109 What is the formal charge on oxygen in the following structure? A. +2 B. 1 C. +1 D. 0 E. 2
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


