Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can someone help me with this two question? ASAP please D Question 5 2 pts The triple point of CO2 is considered to be experimentally
Can someone help me with this two question? ASAP please 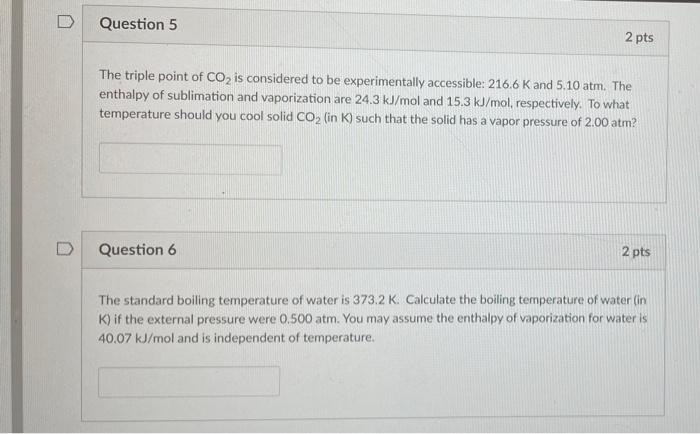
D Question 5 2 pts The triple point of CO2 is considered to be experimentally accessible: 216.6 K and 5.10 atm. The enthalpy of sublimation and vaporization are 24.3 kJ/mol and 15.3 kJ/mol, respectively. To what temperature should you cool solid CO2 (in K) such that the solid has a vapor pressure of 2.00 atm? Question 6 2 pts The standard boiling temperature of water is 373.2 K Calculate the boiling temperature of water in K) if the external pressure were 0.500 atm. You may assume the enthalpy of vaporization for water is 40.07 kJ/mol and is independent of temperature 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


