Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can someone help solve these please? The freezing points of three solutions of glucose in water are shown below. Arrange the solutions in order of
can someone help solve these please? 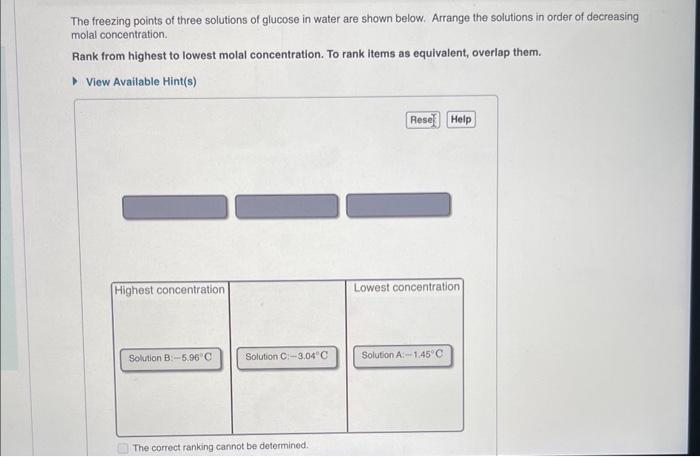
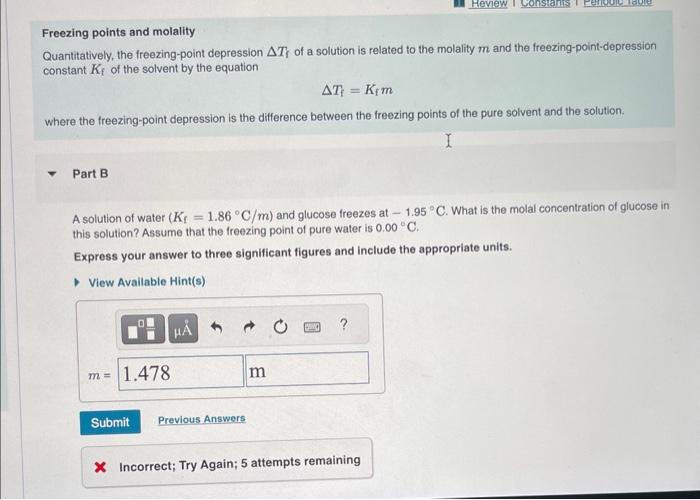
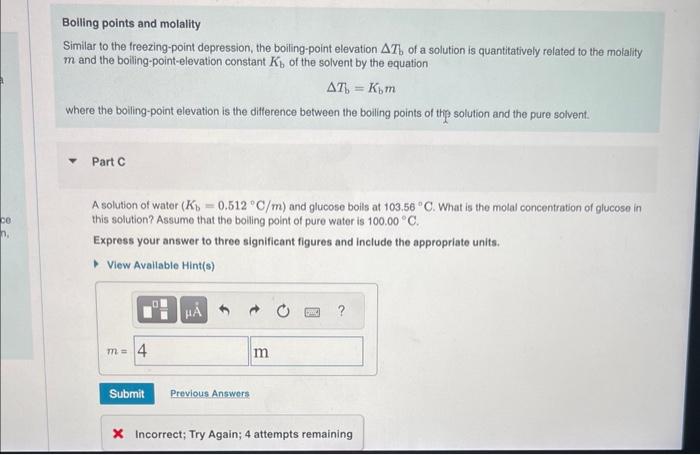
The freezing points of three solutions of glucose in water are shown below. Arrange the solutions in order of decreasing molal concentration. Rank from highest to lowest molal concentration. To rank items as equivalent, overlap them. View Available Hint(s) The correct ranking caninot be determined. Freezing points and molality Quantitatively, the freezing-point depression T, of a solution is related to the molality m and the freezing-point-depression constant Kf of the solvent by the equation Tf=Kfm where the freezing-point depression is the difference between the freezing points of the pure solvent and the solution. Part B A solution of water (Kr=1.86C/m) and glucose freezes at 1.95C. What is the molal concentration of glucose in this solution? Assume that the freezing point of pure water is 0.00C. Express your answer to three significant figures and include the appropriate units. * Incorrect; Try Again; 5 attempts remaining Boiling points and molality Similar to the freezing-point depression, the boiling-point elevation T b of a solution is quantitatively related to the molality m and the boiling-point-elevation constant Kb of the solvent by the equation Tb=Kbm where the boiling-point elevation is the difference between the boiling points of thp solution and the pure solvent. Part C A solution of water (Kb=0.512C/m) and glucose boils at 103.56C. What is the molal concentration of glucose in this solution? Assume that the boiling point of pure water is 100.00C. Express your answer to three significant figures and include the appropriate units. X Incorrect; Try Again; 4 attempts remaining 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


