Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can someone Please answer average bond energy and coordination number of both of sheets but its clear tho i can see very clearly Name: Date:
can someone Please answer average bond energy and coordination number of both of sheets 


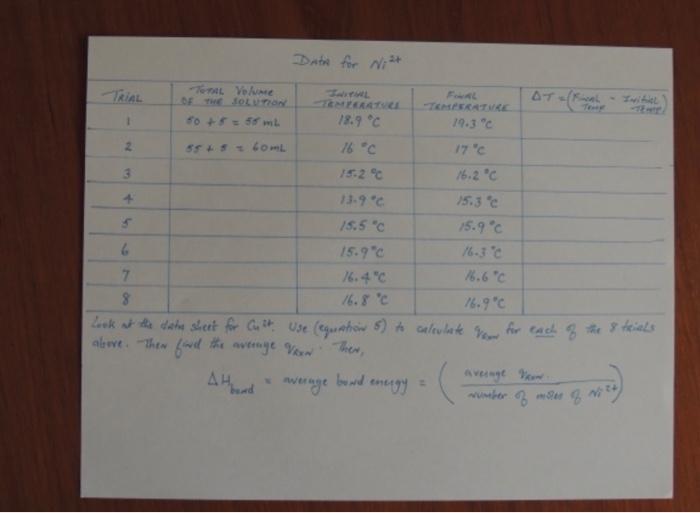




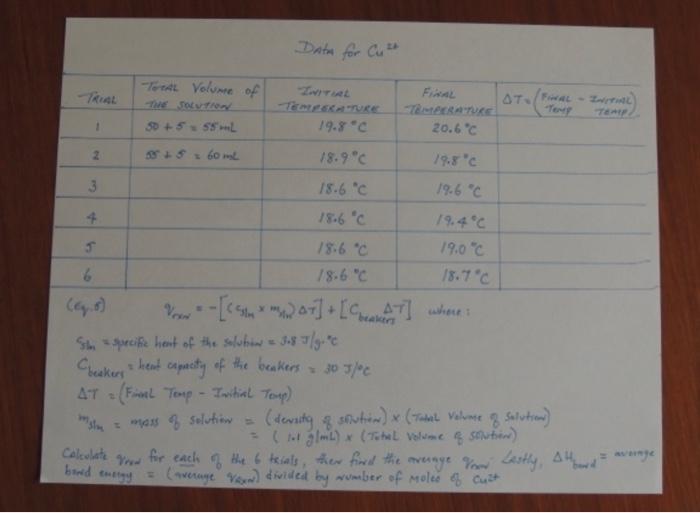
but its clear tho i can see very clearly
Name: Date: Section: Data Sheet: Investigation of Nickel and Copper Coordination Complexes Part B: The Coordination Number of Mass of NK(NO),.611,0 Volume of Deionized 1,0 Concentration of NP Solution Moles Ni TRIAL #Initial TemperatureFinal Temperature Average Bond Energy Coordination Number of Ni? 165 Part B The Coordination Number of Mass of Cu(NOJ, 31,0 Volume of Deionized H,O Concentration of Cut Solution Moles Cut TRIAL #Initial TemperatureFinal Temperature....AH. Average Bond Energy Coordination Number of Cu?' 166 + Ver becker's where = Calculations Equation 5 [* 4) AT] + [Coast AT sin specific heat of the solution - 3.8 J/g. = beakers = heat capacity of the beskev = 30 5/c. AT = (Final Temp- Initial Temp) = mass of solution (density of solution) & (Total Volune of solution) (11 g/mL) * (Tohl volume 3 Solution) data sheet te42 FOR wit Calculate for each of your Calculate the average Vow Calculate Abend = average bowd energy wumber For Cut for each of your trials on your Calculate the average Vou Calculate H = average bowl wurther msin data sheet. Calculate Vas bond energy average rate 8 Malee 8 Data for Wit TRIAL Toms Volume DE THEOLUTION to +55 ml A1-( TEAU 18.9 C -TERATURE 19.3C 1 2 55S COL 16 C 17C 15.2C 16.2C 13.9C 15.3 C 15.5" 15.9C 15.9" 16.3C 7 16.4C 16.6C 8 16.8 C Look at the data shert for Cult use (equation 6) to calculate Now for each of the 8 trials above. Then find the average Von Ther, . avenge wa wumber of g averinge bouwd energy = Data for Cut TOTAL Volume of TRIAL FAL TERATURE 19.8C STO/FINAL INITIAL TERATURE 20.6 C 50 +5.5L Z 05 60ml 18.9C 19.8C 3 18.6C 19.6C + 18.6C 19.4C 18.6 C 19.0C 6 18.6C 18.7C Pow-[**) 04]+[CAT] where Som specific heat of the solution 3.8 7/gore. Checkers head capacity of the beakers - 305/c AT (Final Temp - Initial Toup) men Solution (dersity of Soutien) x (Total Volume of solution) (L) x (The volume soubor) Calcolate for each of the 6 trials, then find the average foron Lastly, alth = (average von divided by wwmber of moles & Cust slim = wenye bond energy Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


