Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can someone please answer b? HBPB(aq)+OH(aq)Yellow 1. HBPB 1+. The acidified form of bromephenol blue, BPB, is yellow and has a strong absorption peak at
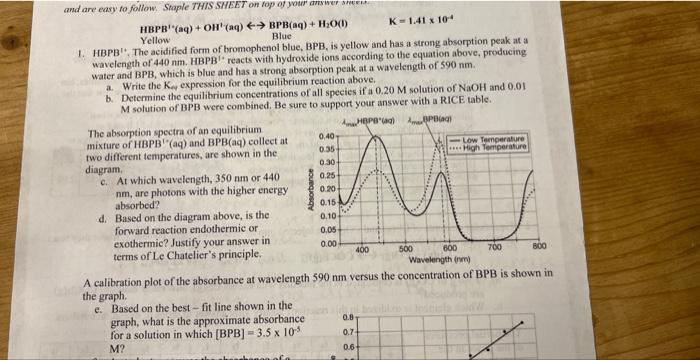
can someone please answer b?
HBPB(aq)+OH(aq)Yellow 1. HBPB 1+. The acidified form of bromephenol blue, BPB, is yellow and has a strong absorption peak at a wavelength of 440mm. HBPB 17 reacts with hydroxide ions according to the equation above, producing water and BPB, which is blue and has a strong absorption peak at a wavelength of 590nm. a. Write the Ked expression for the equilibrium reaction above. b. Determine the equilibrium concentrations of all species if a 0.20M solution of NaOH and 0.01. M solution of BPB were combined. Be sure to support your answer with a RICE table. The absorption spectra of an equilibrium mixture of HBPBt-(aq) and BPB(aq) collect at two different temperatures, are shown in thediagram. c. At which wavelength, 350nm or 440 nm, are photons with the higher energy absorbed? d. Based on the diagram above, is the forward reaction endothermic or exothermic? Justify your answer in terms of Le Chatelier's principle. A calibration plot of the absorbance at wavelength 590nm versus the concentration of BPB is shown the graph. e. Based on the best - fit line shown in the graph, what is the approximate absorbance for a solution in which [BPB]=3.5105 M Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


