Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can someone please check my answers and help me do the last 3 questions? LABORATORY REPORT No. 01: PRECISION, ACCURACY AND SIGNIFICANT FIGURES L DATA
can someone please check my answers and help me do the last 3 questions? 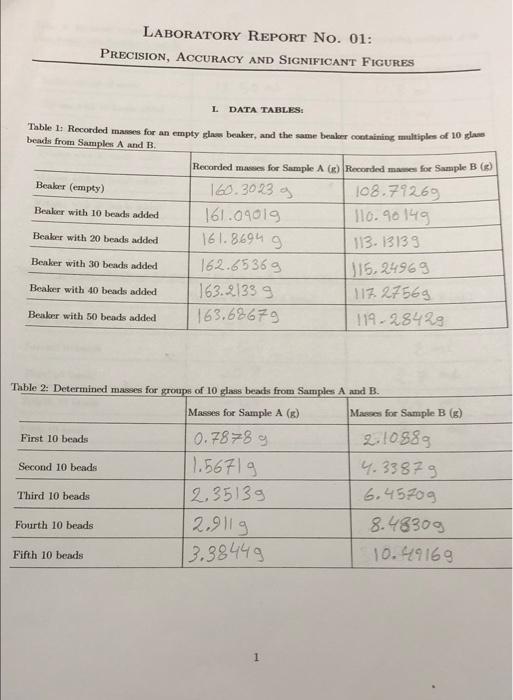
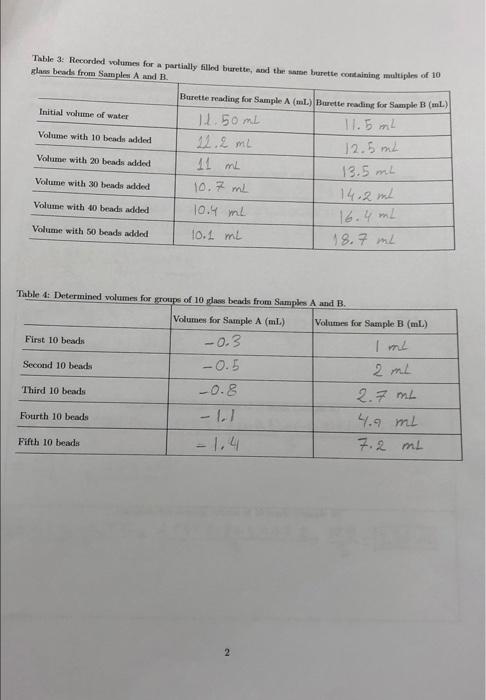

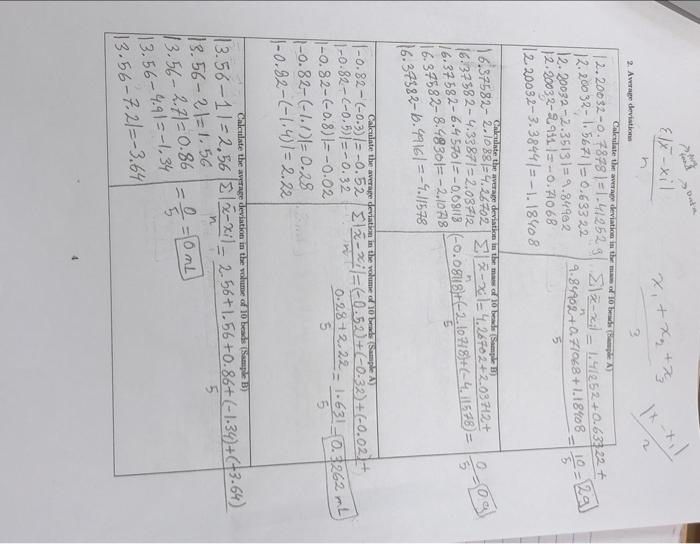



LABORATORY REPORT No. 01: PRECISION, ACCURACY AND SIGNIFICANT FIGURES L DATA TABLES: Table 1: Recorded mames for an empty slow beaker, and the same beaker containing multiples of 10 la beads from Samples A and B. Recorded mes for Sample AG) Recorded mames for Sample B) Beaker (empty) 160.3023 g 108,79269 Beaker with 10 beads added 161.09019 110.90149 Beaker with 20 beads added 161.8694 g 113. 13133 Beaker with 30 beads added 162.65369 315,24969 Beaker with 40 beads added 163.21339 117.27568 Beaker with 50 beads added 163.68679 119-28429 Table 2: Determined masses for groups of 10 glass beads from Samples A and B. Masses for Sample A (8) Masses for Sample B (6) First 10 beads 0.7878 g 2.10889 1.5671 g 4.3387g Third 10 beads 12.35139 6.4570g Fourth 10 beads 2.9119 8.48309 Fifth 10 beads 3.38449 10.49169 Second 10 beads 1 Table 3: Recorded volumes for a partially siled burette, and the same hurette containing multiples of 10 class beads from Samples A and B. Burette reading for Sample A (ml.) Burette reading for Sample B (L) Initial volume of water 11. 50ml 11.5ml Volume with 10 beaded 22.2 ml Volume with 20 beads added 11 mL Volume with 30 beads added 10.7 ml 14.2 ml Volume with 10 beads added 10.4 ml Volume with 50 beads added 10.1 mi 18.7 ml 12.5 ml 13.5 mL 16.4 mL Table 4: Determined volumes for groups of 10 pas beads from Samples A and B. Volumes for Sample A (ml.) Volumes for Sample B (L.) First 10 beads -0.3 Second 10 beach -0.5 2 m2 Third 10 beads -0.8 2.7 ml Fourth 10 beads -1.7 ml Fifth 10 beads 7.2 m 2 IL CALCULATIONS When performing your calculations remember to show your work, Include all equation and its and present your final answers to the correct number of significant figure. Your calculation should follow the format: Show equation Substitute into equation with units Solve equation and prenent final anwer with the correct unit and number of snificant figures 1. Average Values xitxa ta 3 Calculate the average mass of To beads (Sample A) = 0.7878 +1.5671 +2.3513 + 2 911+3.3844 5 11.0016 5 1x=x+4 +2+36 = 2. 200329 . = 2, +%+7+2 Calculate the average maes of 10 headi (Sumple ) = 2.1088 +4.3387 +6.4570+8.4830+10.4916 = 31.87916.375829 5 5 --- Calculate the average volume of 10 beads (Sample A) x= x + xy +28+ xy = (-0,3)+(-0.5)+(-0.8)+(-1.1+(1.4) 4 5 -4.1 5 = -4 1 = (-0.82 mL) Calculate the average volume of To beads (Sample B) = % +2, +7,+ 6 = 4+2+2.754.9+7.2- 15.30 = 3.56 m2 x 4 3 > Elx-xil x,+ x + x 1xx 3 2. A deviation Calculate the average deviation in them of 10 bedste - 2.20032-0-78781=1.412529 3lz-zel 1.4252 +0.63322 12.20032-1.5671|=0.633 22 12. 20032 -2.35131=9.84982 9.8402 +0.71028+ 1.18408 12.2003 2-290115 -0.4068 12.20032-3.3844)=-1.18408 Caleate the average deviation in the mass of 10 beade Sample 16.37582-2.10831-4.26702 312-30 124.2670242.03 H2+ 116.37582-4.33871= 2.03712 16.37582-6.457013-0,09113-o.ogisi162.10 418)+(-4, 11578) = :3-09 16.37582-8.48301= -2.1078 ||6.37582-6.4916] =-9.1578 calculate the average deviation in the volume of 10 beads Sample A) |-0.82-C-0-2) = -0.52 Ela-=-0.5.2+7-0.32)+(-0.02 11-0.82-(-0,5) =-0.32 0.28+222 = 1.631_ 0.3262 mb 11-0.82-(-0.8) 1= -0.02 5 1-0.82-(-1.1)1=0.28 1-0.82-(-1.41=2.22 Calculate the average deviation in the volume of 10 beads (Sampe B 13.56-11-2.56 313-2i! - 2.56+1.56+0.86(-1.34)+(+3.64) ( 13.56-21=1.56 n 0 - = 2 = 10 mL 13.56-2.71=0.86 113.56-4.91=-1.34 113.56-7.21=-3.64 = Data (X-X 1. Standard deviatic - Ceate the standard deviation in the mua 10 bademy Calculate the standard deviation in the molto adimple ) 2037*) = () (12:3513-7.2001 + (2.1)) - 2.200)+(3.38442 200) 3 1.91430BBY+O400562 0,02499169+ 0.505521 + 1.40230330 = 4.326087244 = 1.081521815 1.0829 bax: (++(6.4570-6**=587 + (8.4830-6.39587+(10.9916-6.5559) = 18.207261+ 4.14977647+ 0.00659344 +444024184+16.43980964=43.74376033 10.936 g) EG-21) (6-0.3)-0.832+((-0.59-0.80 ++0.80 -0.97 (((-1.1)-0.80 + ((-1.47-0.82 = 1.2544+1.7424+2.6896+ 3.6864+4.9284= 14.3012 - 3.5753 mL 313.6mb Calculate the standard deviations in the value of 10 beds Sample Calculate the standard deviation in the volume of 10 beds Sample ) (30%) = (1-3.562 +(2-3:56)+( 27-3.56) +(49-3-563+7.2-3.56) 6.5536+2,4336+0.7396 +1.7956+13.2496= 24.772 = 6.193 mL ~ 7 ml 4. Calculated and propagated final quantities Calculate the average deity of the beach (Sample A) mas Yolune Density D=1.0829 3.6ml =0.300555556 30.39/m2 Calculate the propagated percent standard deviation for the density the slow beads (Sample) 161.082 %100) +(3.6X100)? - 1170724 +129600 = 1300324.9/m2 Calculate the propagated absolute standard deviation for the density of the low beads (Sample A) 1300324. = 13003.249/ml 100 Calculate the average density of the glass beads Simple B) Density=mass Volume De 10.9369 = 1.562285714 29/ml 7uL Calculate the propagated percent standard deviation for the density of the glass beads (Sample B) |(10.936X100)2+(7x100)2 = ||45960.96 +490000 ~ 1685960g/mL) Calculate the propagated absolute standard deviation for the density of the glass beads (Sumple B) 1685960 100 = 16859.69/mL 6 TIL EVALUATING YOUR RESULTS 5. Which of the measure values (mass or volume) contributed more to the certainty (standard deviation) in the density? Explain the reason for your answer. 6. Based on your calculated densities, do you think the density of the pas beads in samples A and B are the same? Explain the reason for your answer 7. If the accepted value for the density of the class wed in this experiment in 2.52g mL-', what are the peront errons in your results? 7 






Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


