Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can someone please help me - Kinetics Run I: 1.25 x 10-5 M CV versus 0.020 M NaOH at warm temperature, T=_4045.2C 40-45C Complete the
can someone please help me 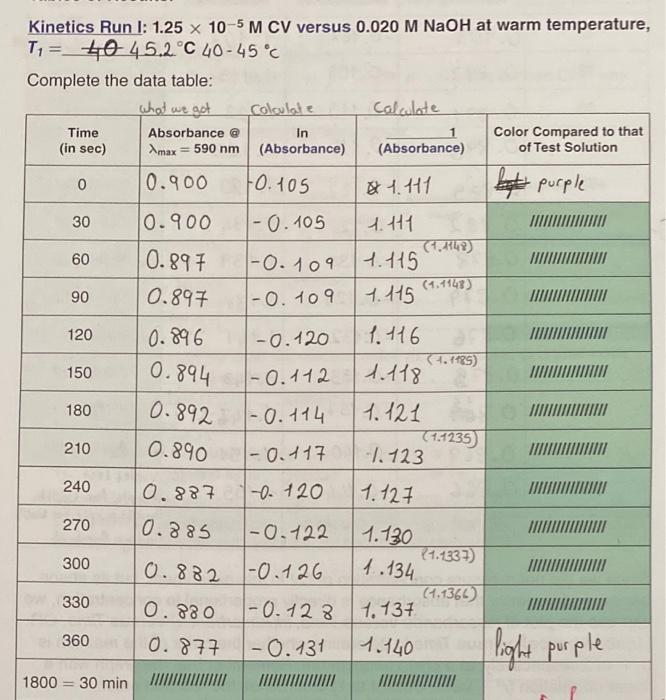
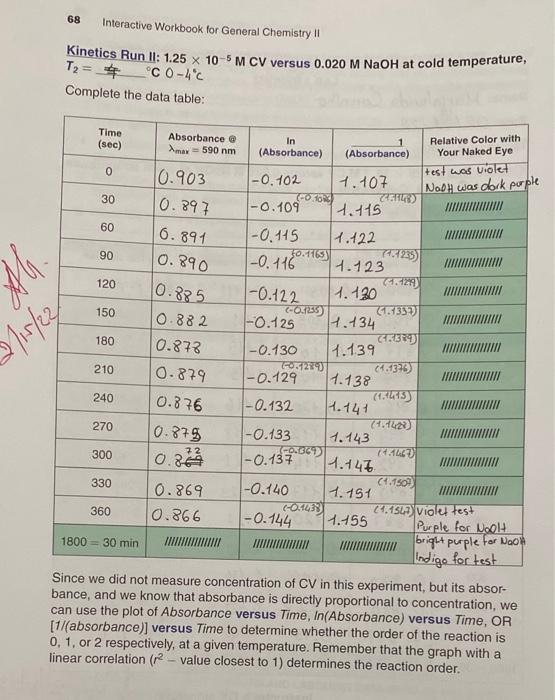
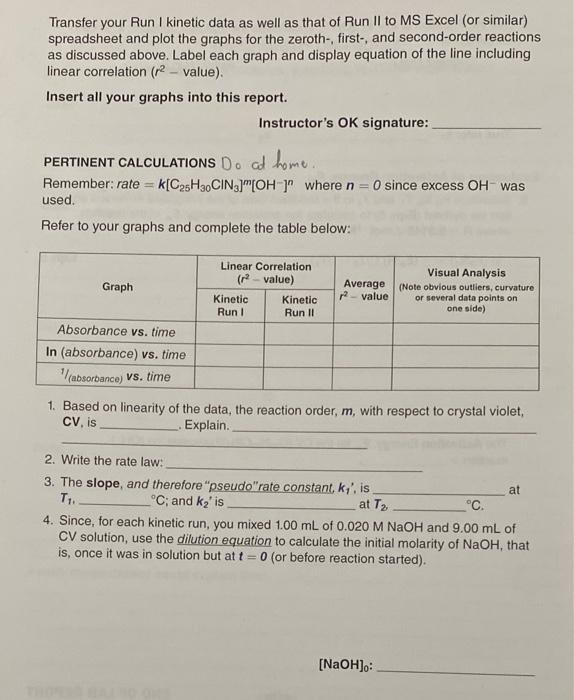

- Kinetics Run I: 1.25 x 10-5 M CV versus 0.020 M NaOH at warm temperature, T=_4045.2C 40-45C Complete the data table: what we got Coloulate Calcolate Time In Color Compared to that (in sec) max = 590 nm (Absorbance) (Absorbance) of Test Solution 0 0.900 Fo.105 1.41 Absorbance @ be purple 30 0.900 1-0. 105 60 1-O. 109 0.897 0.897 t. 111 (4.447) 1.115 (1.1148) 7. 115 90 1-0.109 VIII 120 0.896 -0.120 1.116 (1.1185) 1.118 150 0.894 0.892 1-0. 112 180 1-0.114 1.121 (1.1235 -1.123 210 0.890 1- 0117 240 0.887 -0.120 1.127 270 10.885 -0.122 300 0.882 1.730 (1.337) 1.134. (1.1361) 1-0 126 330 1.137 0.880 0.877 1-0.128 -0.131 360 1.140 light purple 1800 = 30 min III 68 Interactive Workbook for General Chemistry !! Kinetics Run II: 1.25 x 10-5 M CV versus 0.020 M NaOH at cold temperature, T2 = $ C 0-4C Complete the data table: Time (sec) Absorbance @ Amax = 590 nm In (Absorbance) (Absorbance) Relative Color with Your Naked Eye test was violet Nooh was dock porple 0.897 % 6.891 0.890 10.885 Bliste2 0-879 240 0.876 0 10.903 |-0.102 1.107 (-00) 30 -0.109 1.115 60 -0.115 1.122 90 1-0. 116 30.11653 7.123 74.129 120 1-0.122 1.120 150 (-0.135) 4.1337 0.882 -0.125 H.134 180 0.873 -0.130 1.139 210 CO1270 (1.1376 1-0.129 1.138 (1514:15 -0.132 1.141 270 11614232) 10.875 -0.133 1.143 IGOROUD 7247 300 0.84 1-0.137 1.145 330 (1.1509 0.869 1-0.140 1.151 COM 360 0.866 2461547) Violet test 1-0.144 1.155 Purple for Woolt 1800 = 30 min bright purple for Noon Indigo for test Since we did not measure concentration of CV in this experiment, but its absor- bance, and we know that absorbance is directly proportional to concentration, we can use the plot of Absorbance versus Time, In(Absorbance) versus Time, OR [1/(absorbance)] versus Time to determine whether the order of the reaction is 0, 1, or 2 respectively, at a given temperature. Remember that the graph with a linear correlation (2-value closest to 1) determines the reaction order. Transfer your Run I kinetic data as well as that of Run II to MS Excel (or similar) spreadsheet and plot the graphs for the zeroth-, first-, and second-order reactions as discussed above. Label each graph and display equation of the line including linear correlation (2 value) Insert all your graphs into this report. Instructor's OK signature: O since excess OH was PERTINENT CALCULATIONS D. at home Remember: rate = k[C25H3.CIN)"OH)" where n used. Refer to your graphs and complete the table below: Graph Linear Correlation (r-value) Kinetic Kinetic Run Run 11 Average value Visual Analysis (Note obvious outliers, curvature or several data points on one side) Absorbance vs. time In (absorbance) vs. time absorbance) vs. time 1. Based on linearity of the data, the reaction order, m, with respect to crystal violet, CV, is Explain. 2. Write the rate law: 3. The slope, and therefore "pseudo"rate constant, ky is at T1. C; and ka' is at T C. 4. Since, for each kinetic run, you mixed 1.00 mL of 0.020 M NaOH and 9.00 mL of CV solution, use the dilution equation to calculate the initial molarity of NaOH, that is, once it was in solution but at t = 0 (or before reaction started). [NaOH).: 70 Interactive Workbook for General Chemistry 5. Use the Equation 3, k = k17[OH ), to calculate the true rate constant k, at each temperature: Kinetic Run/ Kinetic Run // True rate: kz Evaluation of Activation Energy, E 6. Substitute ky, kz, T, and T, in the modified form of the integrated Arrhenius equa- tion we derived (Egn. 6) to calculate the activation energy, E, of the reaction, where R = 8.3145 J.mol-1K-1: In (kz/k) = -(ER) (/T2 - 1/T1) Egn. 6 The Activation Energy, E, for the CV (C2SH,CIN, hydroxide ion (OH) reaction = --000- END OF LAB REPORT 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


