Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can someone please help me with these questions? I tried solving it but my answers are incorrect. Thank you Determine the pH of an aqueous
Can someone please help me with these questions? I tried solving it but my answers are incorrect. Thank you 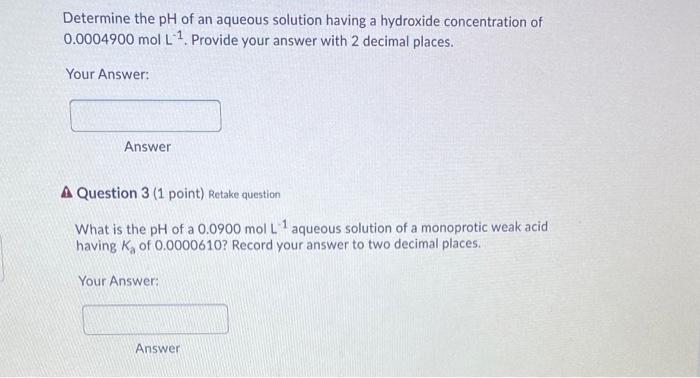
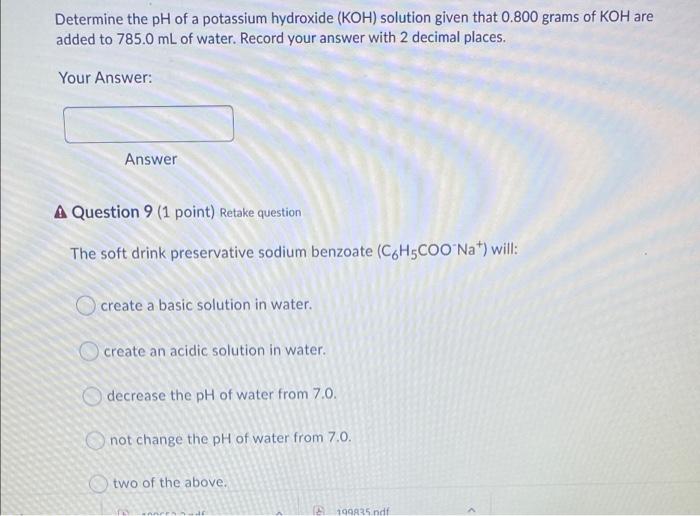

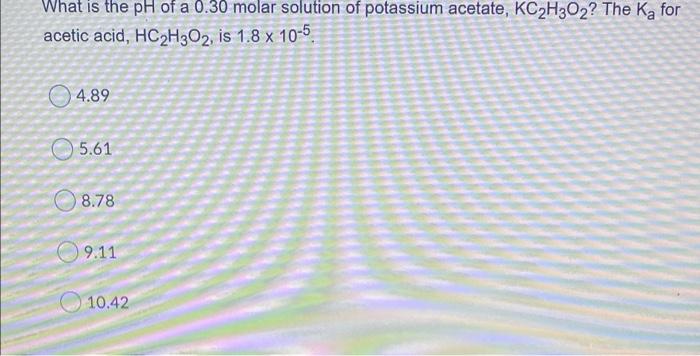

Determine the pH of an aqueous solution having a hydroxide concentration of 0.0004900 mol L. 1. Provide your answer with 2 decimal places. Your Answer: Answer A Question 3 (1 point) Retake question What is the pH of a 0.0900 mol L 1 aqueous solution of a monoprotic weak acid having K of 0.0000610? Record your answer to two decimal places. Your Answer: Answer Determine the pH of a potassium hydroxide (KOH) solution given that 0.800 grams of KOH are added to 785.0 mL of water. Record your answer with 2 decimal places. Your Answer: Answer A Question 9 (1 point) Retake question The soft drink preservative sodium benzoate (CoH5COO Nat) will: create a basic solution in water. create an acidic solution in water. decrease the pH of water from 7.0. not change the pH of water from 7.0. two of the above. 194A25 ndf List the following in order of increased pH of their 0.25 M solutions. (1=lowest pH; 5=highest pH). This question will be marked "all or none". NaCl HCI NH3 Ba(OH)2 NHACI What is the pH of a 0.30 molar solution of potassium acetate, KC2H3O2? The Ka for acetic acid, HC2H302, is 1.8 x 10-5 4.89 5.61 8.78 09.11 10.42 Excluding H20, list all the species present in an aqueous solution of NH4Cl and also give the correct comparison of [H3O+] to (OH). O HCI, NH4+, and [H3O+] > [OH-] NHA. CI, NH3, and [OH"] > [H30* O NH4+, HCl, and (OH'] > [H30+) O NHA. CI', and [H3O+] > [OH') O NHA.CI, NH3, and [H30"] > [OH) 




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


