Can someone take a look at the images below and tell me if I am understanding the concepts of ion movement? Thank you!


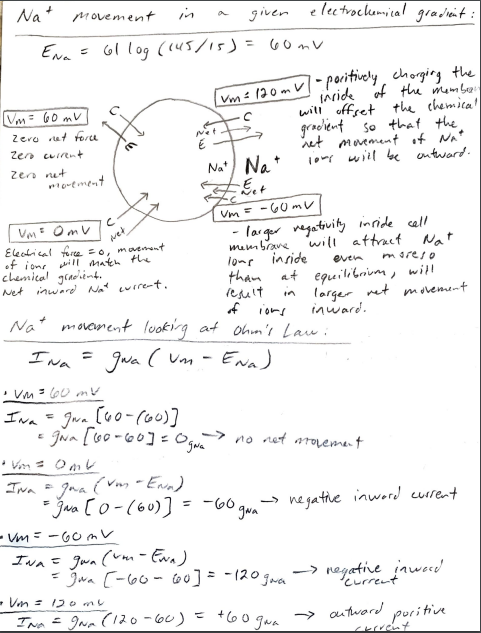
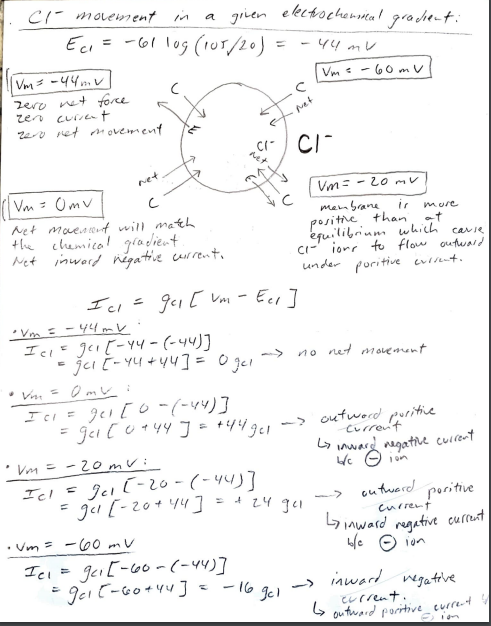
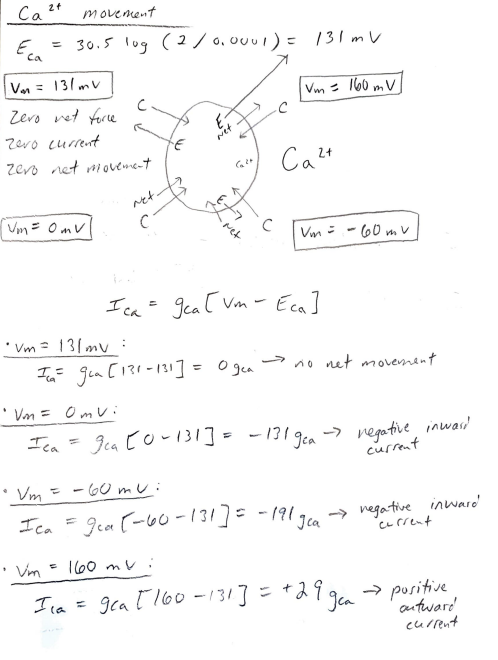
1. In the class presentation you were given the equilibrium potentials for the major four permeable ions for a set of given ionic concentrations in and out of a cell. We worked through the chemical and electrical forces acting on K ions at different membrane potentials and estimated the net ion movement or current. We then used Ohm's law to determine the direction of the K ionic current. In a similar manner use the equilibrium potentials given for Na", Ca?', and Cl to repeat what was shown for K ion movement . Era 6110g (145/15) = 60mV Extracellubri Intracellules 145mm 15mm Ek = 61 10g (5/145). -89m. V k! mm 145mm ECI=-61167(105/20) = -44m2 C- 20mm Eca = 30.5 109( 370.0001) = 13/04 Calt O. Ocol MM Nat / 2 mm Kt movement in a given electrochemical grawient : EK = 011g 5/145) = -89 mV - twet K* kt Net E (Un>Ex) -larger negativity inside the Vm= -89V Vm = -1200V I membrane will attract Kt ione even more than at equilibrium to go Zero Net Force inside the cell. Negative current results Zero current in not movement of our inward. zen net movement Vm = Omy - electrical force = 0, so Net movement of ion will match Vm=-60mul the chemical gradient. Net entward current gets larger. The attractive force of krone outword, poritive current. to into the cell will be inward 3 but less than at -89nr. molemment = outward Outward, positive cuire Net kt movement by looking at Chn's Lawi I=9* (Vm ) gx(vm - Ex) (when (when Exto) ) ontward (+ current) slupe = 9 (conductance) inward (- current) EK e Ek) Ik = gk (um Vm = -89 mV Ik = gk (-89-(-89] 9k (-89 +89] = 10 net movement - no Vm = -60 mv Ik = 9K [-60 - (-89)] 94 [-60+89] = + 29 gk outward, puritive [ Vm =-120ml Ik gk [-120 - (-89)] = 96 [-120+89] = -319k - negative inward - um = Omv Ik=gk[o-(-89)] = $k [0789] = - outward positive current. current +89 gk Nat movement in a given electrockemical gradient : Ena = 61 log (145/15) = 60 mv [Vm= 60 mV Zero net force Zencuit zero net [Vm= 120 mV] - poritively chorging the inside of the member will offset the chemical gradient so that the net movement of Nat Nat Nat ions will be outward. we E be pement Um = -60 mv - larger negativity inside cell membrane will attract wet ever marelo of ions ion! c Vm= Omv Electrical force = 0, movement Nat will match the lor inside chemical gradient tham at equilibrium, will Net inward Nat current. result larger net movement of inward. Nat movement looking at Ohm's Law: Ina = gwa (um - Ena) Vm= 100 m Ina = gora [oo-160)] gora [60-60] =Ogwa no net solement Von Omt TV gna (van - Enn) - gra [o-(60)] negative inword current - Um-60ml In a=gwa (won-Ewa) = Iowa [-60-60] = -120gwa - negative inwood = -> - Vin Domu Ina = gwa (120-60) = +60gwa E =-60 gwa E autward poritive cl- movement a given electrochemnital grachent Ecr=-601 10g (105/20) = -44 mv log Ume - 60 mv C |Vm= -44ml zero net force zero current 2010 red movement ci CI net 2 Vm= Omv Vm= -20 MV membrane ir mure Net movenant will match ot positive than the chemical gradient equilibrium which cause Net inward negative corrento ci- ions to flow outward under puritive current. Ici = guc vm - Eer] Vm = -44mL I els goo{-44-(-44)] =ju -44 +44] = Ojal no net movement negatire current outward I c = gelto -(-44)] gico +44 J = +44 gel out word puritive Current inward Vm = -20mu: Icl = Jul -20-(-44)] ga (-20+44] = + 24 gen Ly inward regative current Vm= -60 mv ble ion Ici = gui[-60-(-44)] ger(- = geil-60+44] = -16 gel inward negative G> outward porrhive cure current. Curren poritive . Ca 2+ movement Eca 30.5 oz (2/ 0, 00 0 ) = 131 mv Va = 131 mv Vm = 160 mv Zero net force zero current zero net movement Ca 2+ E wet 2 Net. Me4 [Um = omv C Vm = -60mv I ca=gea [vm - Eca - 131mv : Ia gia [131-131] = Ogen no net movement Vm = Vm = Omvi Ica gea EO-131] = -131 gea - negative inward current Vm = -60 mu: Ica = gea 5-60-131] = -191 go Ica negative inware current Vm 1600 ml I la = gea[160-131] = +29 gen positive ] current











