Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can u show me the answer including all the calculations for it? thank you Intercompany transactions between Pew Corporation and Sat Corporation, its 80 percent-owned
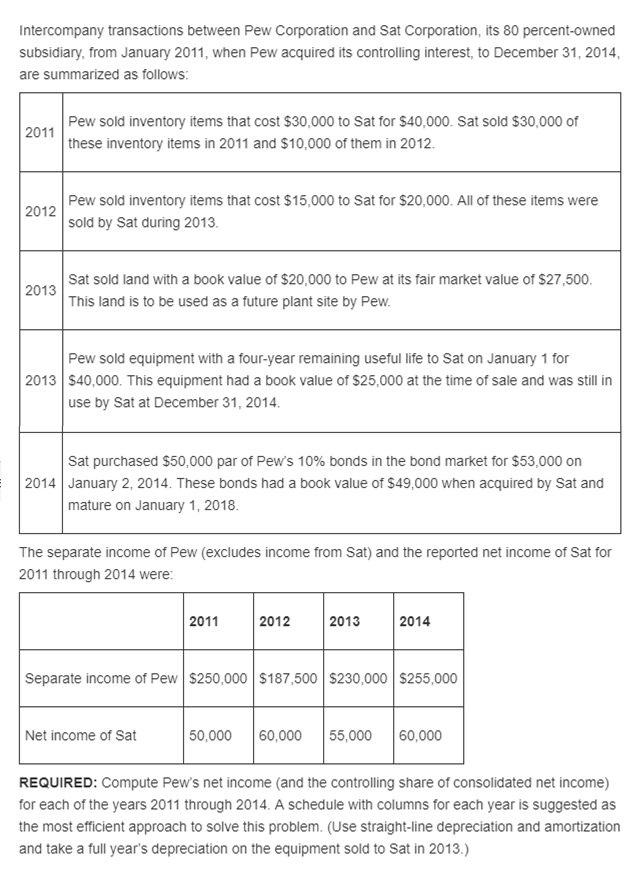
can u show me the answer including all the calculations for it? thank you
Intercompany transactions between Pew Corporation and Sat Corporation, its 80 percent-owned subsidiary, from January 2011, when Pew acquired its controlling interest, to December 31, 2014 are summarized as follows Pew sold inventory items that cost $30,000 to Sat for $40,000. Sat sold $30,000 of these inventory items in 2011 and $10,000 of them in 2012 2011 Pew sold inventory items that cost $15,000 to Sat for $20,000. All of these items were sold by Sat during 2013 2012 Sat sold land with a book value of $20,000 to Pew at its fair market value of $27,500 This land is to be used as a future plant site by Pew 2013 Pew sold equipment with a four-year remaining useful life to Sat on January 1 for 2013 $40,000. This equipment had a book value of $25,000 at the time of sale and was still in use by Sat at December 31, 2014 Sat purchased $50,000 par of Pew's 10% bonds in the bond market for $53,000 on 2014 January 2, 2014. These bonds had a book value of $49,000 when acquired by Sat and mature on January 1, 2018 The separate income of Pew (excludes income from Sat) and the reported net income of Sat for 2011 through 2014 were 2011 2012 2013 2014 Separate income of Pew $250,000 $187,500 $230,000 S255,000 Net income of Sat 50,000 60,000 55,000 60,000 REQUIRED: Compute Pew's net income (and the controlling share of consolidated net income) for each of the years 2011 through 2014. A schedule with columns for each year is suggested as the most efficient approach to solve this problem. (Use straight-line depreciation and amortization and take a full year's depreciation on the equipment sold to Sat in 2013.)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


