Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can uou double check the work i have done, and help me figure out the last table please PROCEDURE CHECK ALL GLASSWARE!!! For this experiment,
can uou double check the work i have done, and help me figure out the last table please 

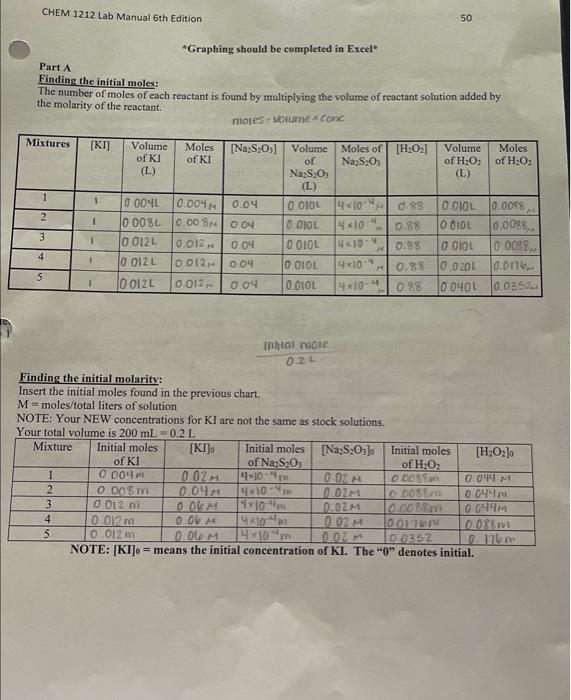
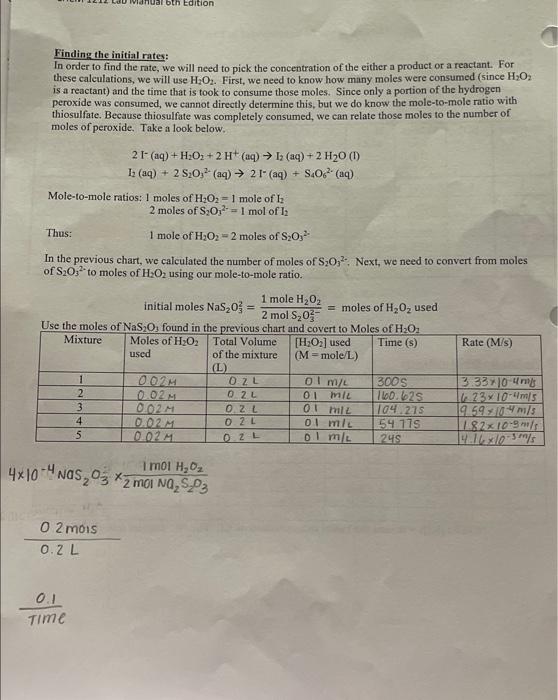
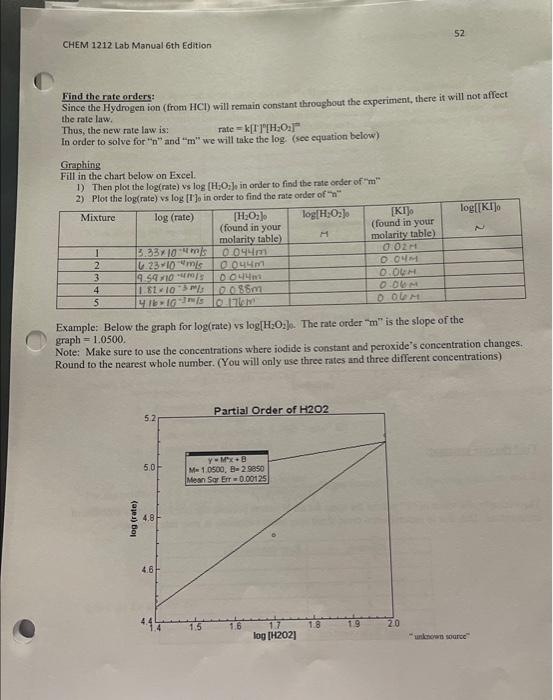 PROCEDURE CHECK ALL GLASSWARE!!! For this experiment, use the table below (Table 1) to help you prepare your mixtures. NOTE: Contamination can be a real problem for this experiment. Pipettes meant for a particular solution should be used only for that specific solution. The beakers should be clean and dry before each kinetics run. BEFORE STARTING PART A, start your warm-water bath and cold-water bath for part B. Fill the beaker about 1/3 full of water. Part A: Determining the Rate Law for the Reaction of Iodide and Hydrogen Peroxide X Obtain a 250mL beaker. Ensure that the inside of the beaker is clean and dry. 4. Measure the volumes of each solution using a graduated cylinder. Dispense the volumes of water, Na2S2O3HCl,Kl and Starch for Mixture 1 according to Table 1 (in that specific order) into the beaker at room temperature (measure and record the temperature of the room on the Report Sheet). Use a stir rod to mix the contents well. 3. While stiring the mixture, carefully and quickly add H2O2 for Mixture 1 according to Table 1 . Immediately start the timer once the peroxide has been added. After about 10 seconds, the mixtures should be homogenous, and you may stop stirring. The addition of H2O2 starts the reaction. 4. Once the blue/black color appears, stop the timer and record the time to the nearest second on the report sheet. The time of the color change may vary between 30 seconds until approximately 800 seconds. If any solution takes longer than this, then there may be something wrong with your solutions. 5. Pour the solution into the waste container. G. Rinse the beaker with tap water and distilled water and dry the beaker. 7. Repeat Steps 14 for all other mixtures. Stock solution Concentrations concentration of KI I concentration of Na2S2O3O.04 concentration of H2O20.88 Part A: Effect of Concentration Room Temperature: Room temp: 19.6C 1) 22.3C 2) 20.9C 3) 216C 4) 21.1C 5) 219C "Graphing should be completed in Excel" Part A Finding the initial moles: The number of moles of each reactant is found by multiplying the volume of reactant solution added by the molarity of the reactant. mores=voiumexrone Inhat note 0.2.1 Finding the initial molarity: Insert the initial moles found in the previous chart. M= molesitotal liters of solution NOTE: Your NEW concentrations for KI are not the same as stock solutions. Your total vnluma is nmimn I. NUIE: [KI]0= means the initial concentration of KI. The " 0+4 denotes initial. Finding the initial rates: In order to find the rate, we will need to pick the concentration of the either a product or a reactant. For these caleulations, we will use H2O2. First, we need to know how many moles were consumed (since H2O2 is a reactant) and the time that is took to consume those moles. Since only a portion of the hydrogen peroxide was consumed, we cannot direetly determine this, but we do know the mole-to-mole ratio with thiosulfate. Because thiosulfate was completely consumed, we can relate those moles to the number of moles of peroxide. Take a look below. 2I(aq)+H2O2+2H+(aq)I2(aq)+2H2O(i)I2(aq)+2S2O32(aq)2I(aq)+S4O62(aq) Mole-to-mole ratios: 1 moles of H2O2=1 mole of H2 2 moles of S2O32=1mol2Il2 Thus: 1 mole of H2O2=2 moles of S2O32. In the previous chart, we calculated the number of moles of S2O32. Next, we need to convert from moles of S2O32 to moles of H2O2 using our mole-to-mole ratio. initial moles NaS2O32=2molS2O321moleH2O2= moles of H2O2 used 4104NaS2O32mOINa2S2O31molH2O2 0.2L02moss Time0.1 Find the rate orders: Since the Hydrogen ion (from HCl) will remain constant throughout the experiment, there it will not affect the rate law. Thus, the new rate law is: rate =k[I]9[H2O2]= In order to solve for " nH and " " m " we will take the log. (see equation below). Giraphing Fill in the chart below on Excel. 1) Then plot the log(rate) vs log[H2O2], in order to find the rate order of "m" 2) Plot the low(rate) vs lod [ITl in order to find the rate order of h Example: Below the graph for log(rate)vlog[H2O2b. The rate order " m " is the slope of the graph =1.0500 Note: Make sure to use the concentrations where iodide is constant and peroxide's concentration changes. Round to the nearest whole number. (You will only use three rates and three different concentrations) PROCEDURE CHECK ALL GLASSWARE!!! For this experiment, use the table below (Table 1) to help you prepare your mixtures. NOTE: Contamination can be a real problem for this experiment. Pipettes meant for a particular solution should be used only for that specific solution. The beakers should be clean and dry before each kinetics run. BEFORE STARTING PART A, start your warm-water bath and cold-water bath for part B. Fill the beaker about 1/3 full of water. Part A: Determining the Rate Law for the Reaction of Iodide and Hydrogen Peroxide X Obtain a 250mL beaker. Ensure that the inside of the beaker is clean and dry. 4. Measure the volumes of each solution using a graduated cylinder. Dispense the volumes of water, Na2S2O3HCl,Kl and Starch for Mixture 1 according to Table 1 (in that specific order) into the beaker at room temperature (measure and record the temperature of the room on the Report Sheet). Use a stir rod to mix the contents well. 3. While stiring the mixture, carefully and quickly add H2O2 for Mixture 1 according to Table 1 . Immediately start the timer once the peroxide has been added. After about 10 seconds, the mixtures should be homogenous, and you may stop stirring. The addition of H2O2 starts the reaction. 4. Once the blue/black color appears, stop the timer and record the time to the nearest second on the report sheet. The time of the color change may vary between 30 seconds until approximately 800 seconds. If any solution takes longer than this, then there may be something wrong with your solutions. 5. Pour the solution into the waste container. G. Rinse the beaker with tap water and distilled water and dry the beaker. 7. Repeat Steps 14 for all other mixtures. Stock solution Concentrations concentration of KI I concentration of Na2S2O3O.04 concentration of H2O20.88 Part A: Effect of Concentration Room Temperature: Room temp: 19.6C 1) 22.3C 2) 20.9C 3) 216C 4) 21.1C 5) 219C "Graphing should be completed in Excel" Part A Finding the initial moles: The number of moles of each reactant is found by multiplying the volume of reactant solution added by the molarity of the reactant. mores=voiumexrone Inhat note 0.2.1 Finding the initial molarity: Insert the initial moles found in the previous chart. M= molesitotal liters of solution NOTE: Your NEW concentrations for KI are not the same as stock solutions. Your total vnluma is nmimn I. NUIE: [KI]0= means the initial concentration of KI. The " 0+4 denotes initial. Finding the initial rates: In order to find the rate, we will need to pick the concentration of the either a product or a reactant. For these caleulations, we will use H2O2. First, we need to know how many moles were consumed (since H2O2 is a reactant) and the time that is took to consume those moles. Since only a portion of the hydrogen peroxide was consumed, we cannot direetly determine this, but we do know the mole-to-mole ratio with thiosulfate. Because thiosulfate was completely consumed, we can relate those moles to the number of moles of peroxide. Take a look below. 2I(aq)+H2O2+2H+(aq)I2(aq)+2H2O(i)I2(aq)+2S2O32(aq)2I(aq)+S4O62(aq) Mole-to-mole ratios: 1 moles of H2O2=1 mole of H2 2 moles of S2O32=1mol2Il2 Thus: 1 mole of H2O2=2 moles of S2O32. In the previous chart, we calculated the number of moles of S2O32. Next, we need to convert from moles of S2O32 to moles of H2O2 using our mole-to-mole ratio. initial moles NaS2O32=2molS2O321moleH2O2= moles of H2O2 used 4104NaS2O32mOINa2S2O31molH2O2 0.2L02moss Time0.1 Find the rate orders: Since the Hydrogen ion (from HCl) will remain constant throughout the experiment, there it will not affect the rate law. Thus, the new rate law is: rate =k[I]9[H2O2]= In order to solve for " nH and " " m " we will take the log. (see equation below). Giraphing Fill in the chart below on Excel. 1) Then plot the log(rate) vs log[H2O2], in order to find the rate order of "m" 2) Plot the low(rate) vs lod [ITl in order to find the rate order of h Example: Below the graph for log(rate)vlog[H2O2b. The rate order " m " is the slope of the graph =1.0500 Note: Make sure to use the concentrations where iodide is constant and peroxide's concentration changes. Round to the nearest whole number. (You will only use three rates and three different concentrations)
PROCEDURE CHECK ALL GLASSWARE!!! For this experiment, use the table below (Table 1) to help you prepare your mixtures. NOTE: Contamination can be a real problem for this experiment. Pipettes meant for a particular solution should be used only for that specific solution. The beakers should be clean and dry before each kinetics run. BEFORE STARTING PART A, start your warm-water bath and cold-water bath for part B. Fill the beaker about 1/3 full of water. Part A: Determining the Rate Law for the Reaction of Iodide and Hydrogen Peroxide X Obtain a 250mL beaker. Ensure that the inside of the beaker is clean and dry. 4. Measure the volumes of each solution using a graduated cylinder. Dispense the volumes of water, Na2S2O3HCl,Kl and Starch for Mixture 1 according to Table 1 (in that specific order) into the beaker at room temperature (measure and record the temperature of the room on the Report Sheet). Use a stir rod to mix the contents well. 3. While stiring the mixture, carefully and quickly add H2O2 for Mixture 1 according to Table 1 . Immediately start the timer once the peroxide has been added. After about 10 seconds, the mixtures should be homogenous, and you may stop stirring. The addition of H2O2 starts the reaction. 4. Once the blue/black color appears, stop the timer and record the time to the nearest second on the report sheet. The time of the color change may vary between 30 seconds until approximately 800 seconds. If any solution takes longer than this, then there may be something wrong with your solutions. 5. Pour the solution into the waste container. G. Rinse the beaker with tap water and distilled water and dry the beaker. 7. Repeat Steps 14 for all other mixtures. Stock solution Concentrations concentration of KI I concentration of Na2S2O3O.04 concentration of H2O20.88 Part A: Effect of Concentration Room Temperature: Room temp: 19.6C 1) 22.3C 2) 20.9C 3) 216C 4) 21.1C 5) 219C "Graphing should be completed in Excel" Part A Finding the initial moles: The number of moles of each reactant is found by multiplying the volume of reactant solution added by the molarity of the reactant. mores=voiumexrone Inhat note 0.2.1 Finding the initial molarity: Insert the initial moles found in the previous chart. M= molesitotal liters of solution NOTE: Your NEW concentrations for KI are not the same as stock solutions. Your total vnluma is nmimn I. NUIE: [KI]0= means the initial concentration of KI. The " 0+4 denotes initial. Finding the initial rates: In order to find the rate, we will need to pick the concentration of the either a product or a reactant. For these caleulations, we will use H2O2. First, we need to know how many moles were consumed (since H2O2 is a reactant) and the time that is took to consume those moles. Since only a portion of the hydrogen peroxide was consumed, we cannot direetly determine this, but we do know the mole-to-mole ratio with thiosulfate. Because thiosulfate was completely consumed, we can relate those moles to the number of moles of peroxide. Take a look below. 2I(aq)+H2O2+2H+(aq)I2(aq)+2H2O(i)I2(aq)+2S2O32(aq)2I(aq)+S4O62(aq) Mole-to-mole ratios: 1 moles of H2O2=1 mole of H2 2 moles of S2O32=1mol2Il2 Thus: 1 mole of H2O2=2 moles of S2O32. In the previous chart, we calculated the number of moles of S2O32. Next, we need to convert from moles of S2O32 to moles of H2O2 using our mole-to-mole ratio. initial moles NaS2O32=2molS2O321moleH2O2= moles of H2O2 used 4104NaS2O32mOINa2S2O31molH2O2 0.2L02moss Time0.1 Find the rate orders: Since the Hydrogen ion (from HCl) will remain constant throughout the experiment, there it will not affect the rate law. Thus, the new rate law is: rate =k[I]9[H2O2]= In order to solve for " nH and " " m " we will take the log. (see equation below). Giraphing Fill in the chart below on Excel. 1) Then plot the log(rate) vs log[H2O2], in order to find the rate order of "m" 2) Plot the low(rate) vs lod [ITl in order to find the rate order of h Example: Below the graph for log(rate)vlog[H2O2b. The rate order " m " is the slope of the graph =1.0500 Note: Make sure to use the concentrations where iodide is constant and peroxide's concentration changes. Round to the nearest whole number. (You will only use three rates and three different concentrations) PROCEDURE CHECK ALL GLASSWARE!!! For this experiment, use the table below (Table 1) to help you prepare your mixtures. NOTE: Contamination can be a real problem for this experiment. Pipettes meant for a particular solution should be used only for that specific solution. The beakers should be clean and dry before each kinetics run. BEFORE STARTING PART A, start your warm-water bath and cold-water bath for part B. Fill the beaker about 1/3 full of water. Part A: Determining the Rate Law for the Reaction of Iodide and Hydrogen Peroxide X Obtain a 250mL beaker. Ensure that the inside of the beaker is clean and dry. 4. Measure the volumes of each solution using a graduated cylinder. Dispense the volumes of water, Na2S2O3HCl,Kl and Starch for Mixture 1 according to Table 1 (in that specific order) into the beaker at room temperature (measure and record the temperature of the room on the Report Sheet). Use a stir rod to mix the contents well. 3. While stiring the mixture, carefully and quickly add H2O2 for Mixture 1 according to Table 1 . Immediately start the timer once the peroxide has been added. After about 10 seconds, the mixtures should be homogenous, and you may stop stirring. The addition of H2O2 starts the reaction. 4. Once the blue/black color appears, stop the timer and record the time to the nearest second on the report sheet. The time of the color change may vary between 30 seconds until approximately 800 seconds. If any solution takes longer than this, then there may be something wrong with your solutions. 5. Pour the solution into the waste container. G. Rinse the beaker with tap water and distilled water and dry the beaker. 7. Repeat Steps 14 for all other mixtures. Stock solution Concentrations concentration of KI I concentration of Na2S2O3O.04 concentration of H2O20.88 Part A: Effect of Concentration Room Temperature: Room temp: 19.6C 1) 22.3C 2) 20.9C 3) 216C 4) 21.1C 5) 219C "Graphing should be completed in Excel" Part A Finding the initial moles: The number of moles of each reactant is found by multiplying the volume of reactant solution added by the molarity of the reactant. mores=voiumexrone Inhat note 0.2.1 Finding the initial molarity: Insert the initial moles found in the previous chart. M= molesitotal liters of solution NOTE: Your NEW concentrations for KI are not the same as stock solutions. Your total vnluma is nmimn I. NUIE: [KI]0= means the initial concentration of KI. The " 0+4 denotes initial. Finding the initial rates: In order to find the rate, we will need to pick the concentration of the either a product or a reactant. For these caleulations, we will use H2O2. First, we need to know how many moles were consumed (since H2O2 is a reactant) and the time that is took to consume those moles. Since only a portion of the hydrogen peroxide was consumed, we cannot direetly determine this, but we do know the mole-to-mole ratio with thiosulfate. Because thiosulfate was completely consumed, we can relate those moles to the number of moles of peroxide. Take a look below. 2I(aq)+H2O2+2H+(aq)I2(aq)+2H2O(i)I2(aq)+2S2O32(aq)2I(aq)+S4O62(aq) Mole-to-mole ratios: 1 moles of H2O2=1 mole of H2 2 moles of S2O32=1mol2Il2 Thus: 1 mole of H2O2=2 moles of S2O32. In the previous chart, we calculated the number of moles of S2O32. Next, we need to convert from moles of S2O32 to moles of H2O2 using our mole-to-mole ratio. initial moles NaS2O32=2molS2O321moleH2O2= moles of H2O2 used 4104NaS2O32mOINa2S2O31molH2O2 0.2L02moss Time0.1 Find the rate orders: Since the Hydrogen ion (from HCl) will remain constant throughout the experiment, there it will not affect the rate law. Thus, the new rate law is: rate =k[I]9[H2O2]= In order to solve for " nH and " " m " we will take the log. (see equation below). Giraphing Fill in the chart below on Excel. 1) Then plot the log(rate) vs log[H2O2], in order to find the rate order of "m" 2) Plot the low(rate) vs lod [ITl in order to find the rate order of h Example: Below the graph for log(rate)vlog[H2O2b. The rate order " m " is the slope of the graph =1.0500 Note: Make sure to use the concentrations where iodide is constant and peroxide's concentration changes. Round to the nearest whole number. (You will only use three rates and three different concentrations)
can uou double check the work i have done, and help me figure out the last table please 





Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


