Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Consider a system consisting of three phases S, L, and G. Follow the sequence of steps (a)-(h) below to derive the conditions for equilibrium
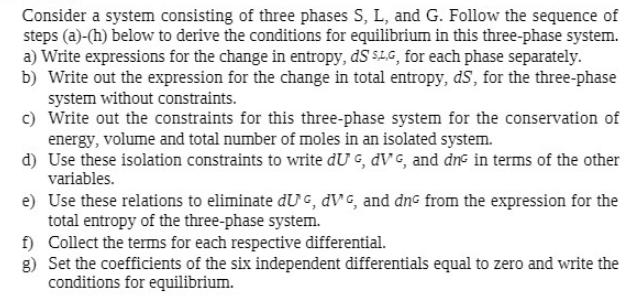
Consider a system consisting of three phases S, L, and G. Follow the sequence of steps (a)-(h) below to derive the conditions for equilibrium in this three-phase system. a) Write expressions for the change in entropy, dS SL,G, for each phase separately. b) Write out the expression for the change in total entropy, ds, for the three-phase system without constraints. c) Write out the constraints for this three-phase system for the conservation of energy, volume and total number of moles in an isolated system. d) Use these isolation constraints to write dU, dVG, and dne in terms of the other variables. e) Use these relations to eliminate du, dVG, and dn from the expression for the total entropy of the three-phase system. f) Collect the terms for each respective differential. g) Set the coefficients of the six independent differentials equal to zero and write the conditions for equilibrium.
Step by Step Solution
★★★★★
3.45 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


