Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can you do this experiment and calculate the result Objective- To determine the enthalpy of vaporization of water using the Clausius-Clapeyron equation. BackGroundInformation- In the
can you do this experiment and calculate the result
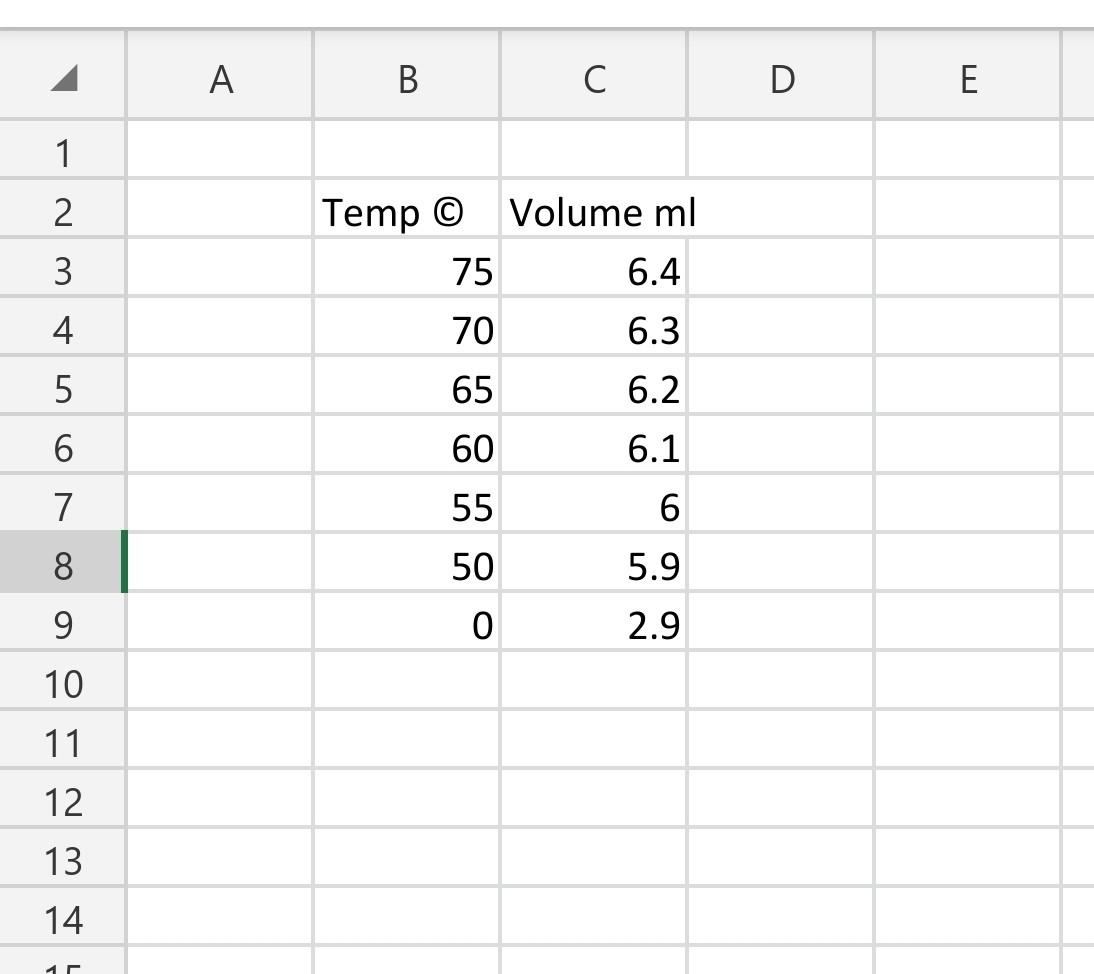

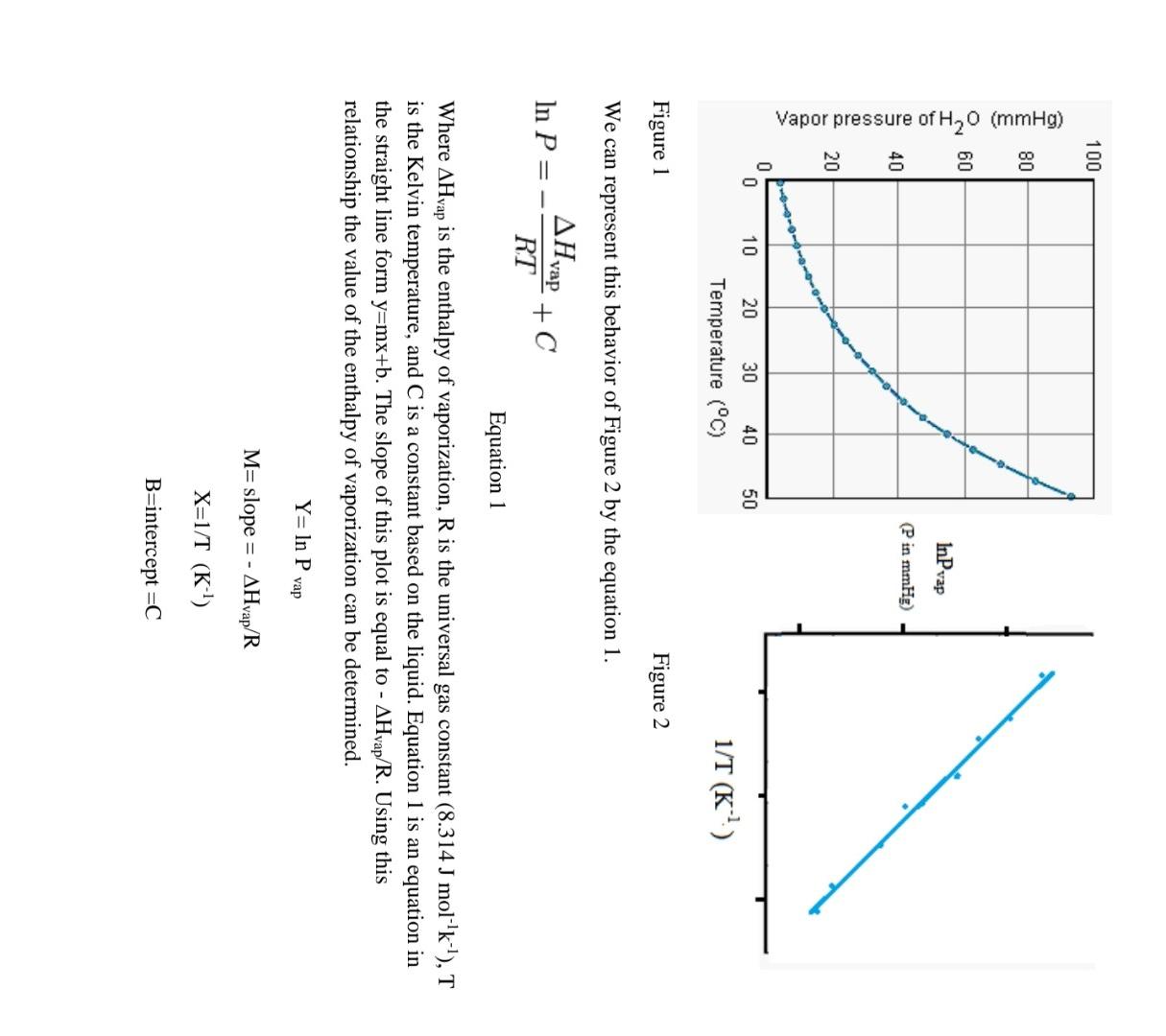
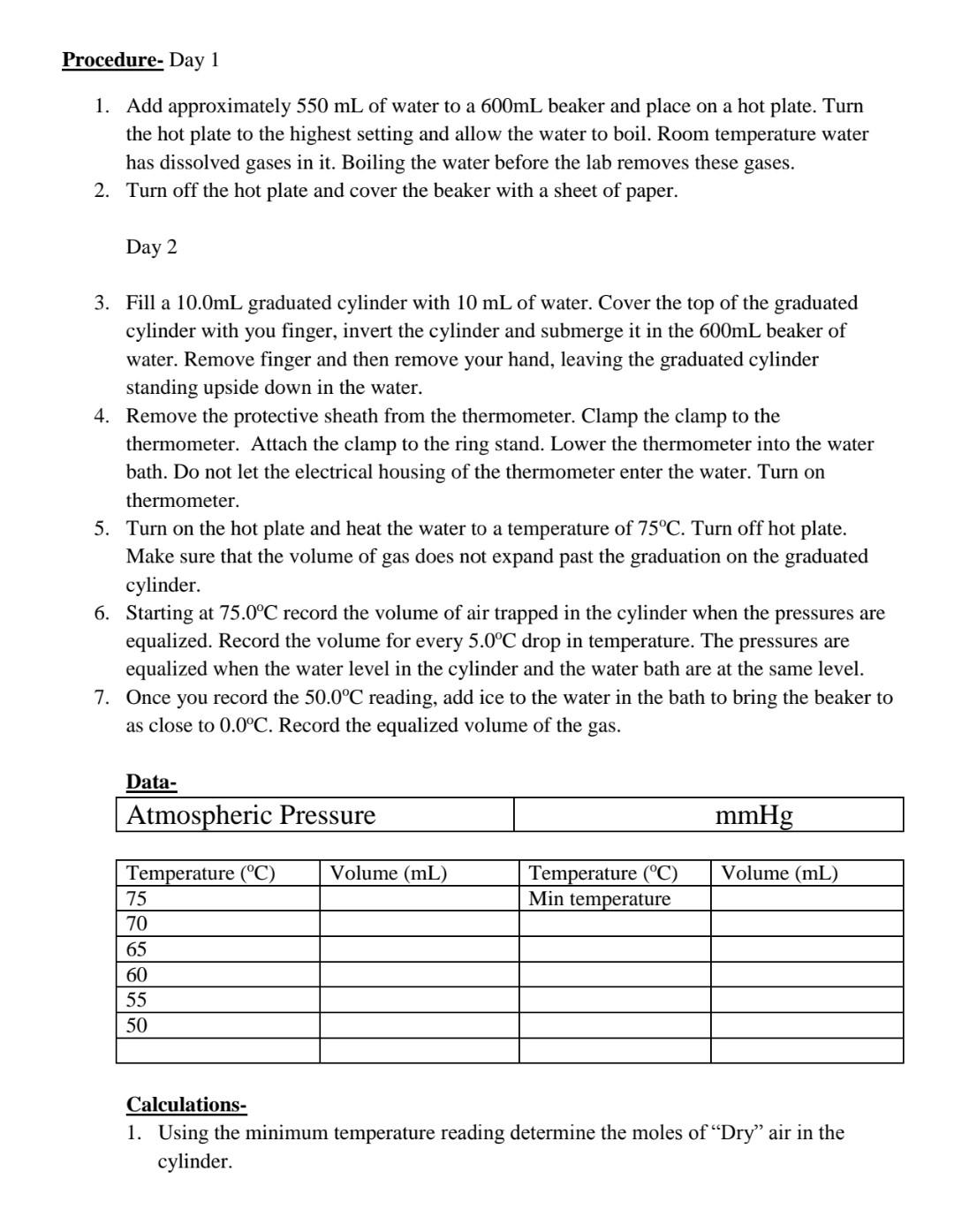

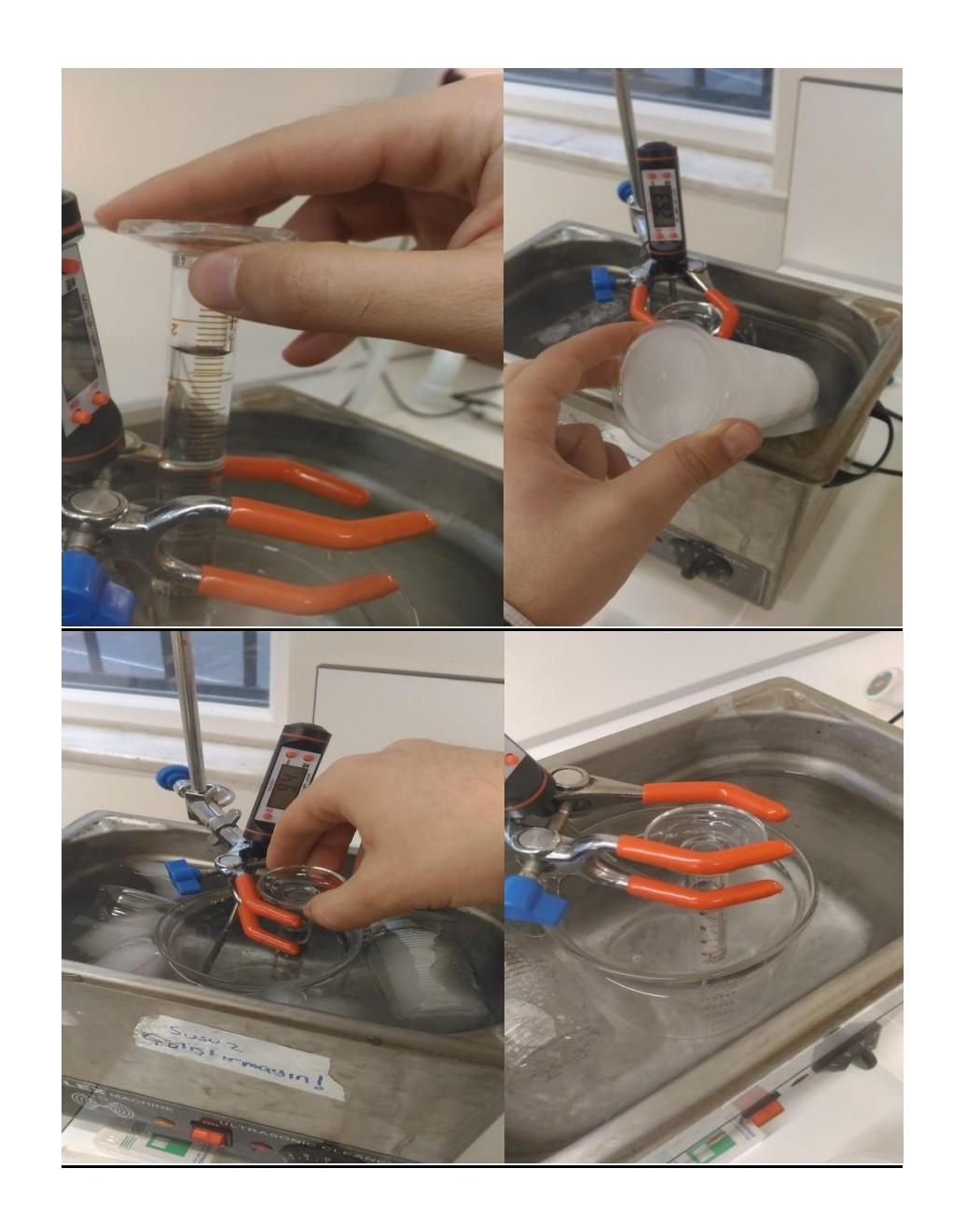

Objective- To determine the enthalpy of vaporization of water using the Clausius-Clapeyron equation. BackGroundInformation- In the experiment you will determine the vapor pressure of a liquid at various temperatures. Vapor pressure is an intensive property, which means it is independent of quantity. This property does change with temperature, the higher the temperature, the greater the vapor pressure. There are several ways to determine vapor pressure. Since, boiling occurs when vapor pressure equals atmospheric pressure. You could change the atmospheric pressure and observe the temperature which a liquid boils. Lowering the pressure lowers the boiling point. This is too easy. Actually we do not have the equipment to determine the vapor pressure this way. This experiment will use a small amount of air trapped in an inverted 10.0mL graduated cylinder immersed in a beaker of water. The water temperature will range from 75.0C down to 50.0C and then lowered to 0.0C by adding a large quantity of ice to the beaker. As the water bath cools, the temperature and volume of the gas will be recorded every 5.0C. In order to minimize the error, the measuring of the volume of gas should only be made if the pressure inside the graduated cylinder and the atmosphere are equal. The graduated cylinder will be raise to allow the water level inside the cylinder and the water bath to be equal. The pressure inside the cylinder and outside the cylinder will be equal. Only then can the volume of the gas inside be measured. The most crucial measurement for this experiment is the 0.0C, where the assumed vapor pressure of water is zero. Any error measuring here will cause an enormous error later in the lab. The Clausius Clapeyron Equation is a mathematical relationship relating the vapor pressure of a liquid to the temperature of the liquid. When vapor pressure is plotted against temperature, a nonlinear trend is observed, as shown in figure 1 . We find that a straight line can be obtained by plotting ln(Pvap) versus 1/T, where T is the Kelvin temperature as shown in figure 2. F1gure I Figure 2 We can represent this behavior of Figure 2 by the equation 1 . lnP=RTHvap+C Equation 1 Where Hvap is the enthalpy of vaporization, R is the universal gas constant (8.314Jmol1k1),T is the Kelvin temperature, and C is a constant based on the liquid. Equation 1 is an equation in the straight line form y=mx+b. The slope of this plot is equal to Hvap/R. Using this relationship the value of the enthalpy of vaporization can be determined. Y=lnPvapM=slope=Hvap/RX=1/T(K1)B=intercept=C Procedure- Day 1 1. Add approximately 550mL of water to a 600mL beaker and place on a hot plate. Turn the hot plate to the highest setting and allow the water to boil. Room temperature water has dissolved gases in it. Boiling the water before the lab removes these gases. 2. Turn off the hot plate and cover the beaker with a sheet of paper. Day 2 3. Fill a 10.0mL graduated cylinder with 10mL of water. Cover the top of the graduated cylinder with you finger, invert the cylinder and submerge it in the 600mL beaker of water. Remove finger and then remove your hand, leaving the graduated cylinder standing upside down in the water. 4. Remove the protective sheath from the thermometer. Clamp the clamp to the thermometer. Attach the clamp to the ring stand. Lower the thermometer into the water bath. Do not let the electrical housing of the thermometer enter the water. Turn on thermometer. 5. Turn on the hot plate and heat the water to a temperature of 75C. Turn off hot plate. Make sure that the volume of gas does not expand past the graduation on the graduated cylinder. 6. Starting at 75.0C record the volume of air trapped in the cylinder when the pressures are equalized. Record the volume for every 5.0C drop in temperature. The pressures are equalized when the water level in the cylinder and the water bath are at the same level. 7. Once you record the 50.0C reading, add ice to the water in the bath to bring the beaker to as close to 0.0C. Record the equalized volume of the gas. Calculations- 1. Using the minimum temperature reading determine the moles of "Dry" air in the cylinder. nair=PV/RT ***Calculation 2-4 should be performed using Microsoft excel, or another spreadsheet program. 2. Using the moles of "Dry" air determine the partial pressure of air in the cylinder at each temperature. Pair=nairRT/V 3. Since the pressure in the cylinder was equalized with the atmosphere before measuring, we can determine the pressure from the water vapor by subtracting the calculated pressure of the dry air from the atmospheric pressure. 4. Plot a graph of ln Pvap vs. 1/T. Using the trend-line function, find the slope of the best fit line. Also record R2, which indicates how close the data is to the straight best fit line. R2 equal to 1.00 is a straight line. Deviations from this indicates poor quality data. 5. Using the slope calculate the enthalpy of vaporization. Hvap=Rslope Questions- 1. What was the % error of your experiment? Hvap if water is 40.65kJmol1. What can be possible reasons for the error? 2. The theoretical Hvap for hexane is 28.88kJ/mol. In terms of intermolecular forces, explain the difference between the heat of vaporization of water and hexane. 3. Compare the results in ideal and real case where the theoretical values are calculated from Van der Waals equations of state. For example; You can compare your results by doing the same calculations by using other equations of state. For example; For van der waals equation of state; atm* liter2/mol2,b=0.0366L/mol for air, you should select R gas constant according to these units. You should use excell and you can use goal seek option to make the necessary calculations. Objective- To determine the enthalpy of vaporization of water using the Clausius-Clapeyron equation. BackGroundInformation- In the experiment you will determine the vapor pressure of a liquid at various temperatures. Vapor pressure is an intensive property, which means it is independent of quantity. This property does change with temperature, the higher the temperature, the greater the vapor pressure. There are several ways to determine vapor pressure. Since, boiling occurs when vapor pressure equals atmospheric pressure. You could change the atmospheric pressure and observe the temperature which a liquid boils. Lowering the pressure lowers the boiling point. This is too easy. Actually we do not have the equipment to determine the vapor pressure this way. This experiment will use a small amount of air trapped in an inverted 10.0mL graduated cylinder immersed in a beaker of water. The water temperature will range from 75.0C down to 50.0C and then lowered to 0.0C by adding a large quantity of ice to the beaker. As the water bath cools, the temperature and volume of the gas will be recorded every 5.0C. In order to minimize the error, the measuring of the volume of gas should only be made if the pressure inside the graduated cylinder and the atmosphere are equal. The graduated cylinder will be raise to allow the water level inside the cylinder and the water bath to be equal. The pressure inside the cylinder and outside the cylinder will be equal. Only then can the volume of the gas inside be measured. The most crucial measurement for this experiment is the 0.0C, where the assumed vapor pressure of water is zero. Any error measuring here will cause an enormous error later in the lab. The Clausius Clapeyron Equation is a mathematical relationship relating the vapor pressure of a liquid to the temperature of the liquid. When vapor pressure is plotted against temperature, a nonlinear trend is observed, as shown in figure 1 . We find that a straight line can be obtained by plotting ln(Pvap) versus 1/T, where T is the Kelvin temperature as shown in figure 2. F1gure I Figure 2 We can represent this behavior of Figure 2 by the equation 1 . lnP=RTHvap+C Equation 1 Where Hvap is the enthalpy of vaporization, R is the universal gas constant (8.314Jmol1k1),T is the Kelvin temperature, and C is a constant based on the liquid. Equation 1 is an equation in the straight line form y=mx+b. The slope of this plot is equal to Hvap/R. Using this relationship the value of the enthalpy of vaporization can be determined. Y=lnPvapM=slope=Hvap/RX=1/T(K1)B=intercept=C Procedure- Day 1 1. Add approximately 550mL of water to a 600mL beaker and place on a hot plate. Turn the hot plate to the highest setting and allow the water to boil. Room temperature water has dissolved gases in it. Boiling the water before the lab removes these gases. 2. Turn off the hot plate and cover the beaker with a sheet of paper. Day 2 3. Fill a 10.0mL graduated cylinder with 10mL of water. Cover the top of the graduated cylinder with you finger, invert the cylinder and submerge it in the 600mL beaker of water. Remove finger and then remove your hand, leaving the graduated cylinder standing upside down in the water. 4. Remove the protective sheath from the thermometer. Clamp the clamp to the thermometer. Attach the clamp to the ring stand. Lower the thermometer into the water bath. Do not let the electrical housing of the thermometer enter the water. Turn on thermometer. 5. Turn on the hot plate and heat the water to a temperature of 75C. Turn off hot plate. Make sure that the volume of gas does not expand past the graduation on the graduated cylinder. 6. Starting at 75.0C record the volume of air trapped in the cylinder when the pressures are equalized. Record the volume for every 5.0C drop in temperature. The pressures are equalized when the water level in the cylinder and the water bath are at the same level. 7. Once you record the 50.0C reading, add ice to the water in the bath to bring the beaker to as close to 0.0C. Record the equalized volume of the gas. Calculations- 1. Using the minimum temperature reading determine the moles of "Dry" air in the cylinder. nair=PV/RT ***Calculation 2-4 should be performed using Microsoft excel, or another spreadsheet program. 2. Using the moles of "Dry" air determine the partial pressure of air in the cylinder at each temperature. Pair=nairRT/V 3. Since the pressure in the cylinder was equalized with the atmosphere before measuring, we can determine the pressure from the water vapor by subtracting the calculated pressure of the dry air from the atmospheric pressure. 4. Plot a graph of ln Pvap vs. 1/T. Using the trend-line function, find the slope of the best fit line. Also record R2, which indicates how close the data is to the straight best fit line. R2 equal to 1.00 is a straight line. Deviations from this indicates poor quality data. 5. Using the slope calculate the enthalpy of vaporization. Hvap=Rslope Questions- 1. What was the % error of your experiment? Hvap if water is 40.65kJmol1. What can be possible reasons for the error? 2. The theoretical Hvap for hexane is 28.88kJ/mol. In terms of intermolecular forces, explain the difference between the heat of vaporization of water and hexane. 3. Compare the results in ideal and real case where the theoretical values are calculated from Van der Waals equations of state. For example; You can compare your results by doing the same calculations by using other equations of state. For example; For van der waals equation of state; atm* liter2/mol2,b=0.0366L/mol for air, you should select R gas constant according to these units. You should use excell and you can use goal seek option to make the necessary calculations
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


