Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can you help me answer question 8 Claustus.Clapeyron Equation - Rudolf Clausius probably didn't have a very shinny nose, but he and Benoit Clapeyron developed
can you help me answer question 8 
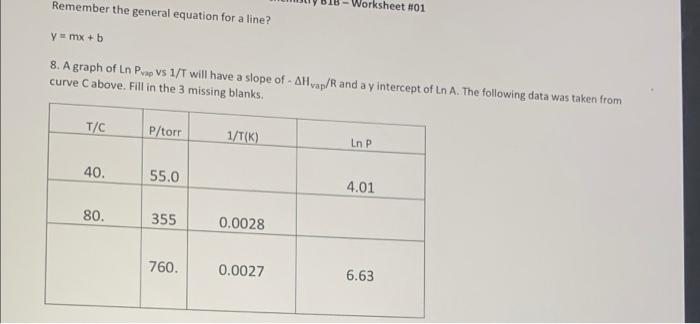
Claustus.Clapeyron Equation - Rudolf Clausius probably didn't have a very shinny nose, but he and Benoit Clapeyron developed the following to account for the exponential increase in vapor pressure with increasing temperature. Pvap = A e- -AH/RT The graph above shows the exponential increase of vapor pressure as temperature increases. Temperature needs to be in Kelvin and the ideal gas constant, R, of 8.314 /mol Kis used. Taking the natural logarithm of both sides gives AHvap! In(Pvap) + In A RT Please continue Remember the general equation for a line? Worksheet #01 Ymx + b 8. A graph of Ln Prap vs 1/T will have a slope of - AH vap/R and a y intercept of Ln A. The following data was taken from curve C above. Fill in the 3 missing blanks T/C P/torr 1/T(K) Ln P 40. 55.0 4.01 80. 355 0.0028 760. 0.0027 6.63 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


