Answered step by step
Verified Expert Solution
Question
1 Approved Answer
can you help me im trying to theroretical yield and percent yeild? Picture on the bottom will give you values. Procedure Part 1 - Reduction
can you help me im trying to theroretical yield and percent yeild? 


Procedure Part 1 - Reduction using tin and hydrochloric acid a) Reaction 1. Place small pieces of tin (3.3g) in a 100mL round bottom flask equipped with a reflux condenser and a magnetic stir bar. 2. Add 3 -nitroacetophenone (1.65g) to the flask, followed by water (24mL) and concentrated HCl(9mL). Concentrated hydrochloric acid causes severe bums. 3. Stir the mixture, and heat the flask to reflux for one hour While this reaction is proceeding, obtain an IR spectrum of the starting material, 3-nitroacetophenone b) Isolation of Crude Product 4. Cool the reaction mixture to room temperature and remove the unreacted tin by suction filtration with a Bchner funnel. (While the reaction is cooling you should start heating Part 2 - Reduction using sodium borohydride a) Reaction 1. Dissolve 3-nitroacetophenone (1.65g) in warm 100% ethanol (20mL) in a 125mL Erlenmeyer flask. 2. Stir the solution using a magnetic stirrer, and cool the flask in an ice bath (the starting material may form a fine precipitate at this point). 3. Add the sodium borohydride (0.45g) in small portions over 5min and stir the reaction mixture at room temperature for 15min. 4. Add water (15mL) to the mixture, nd heat it to its boiling point. b) Isolation of Product 5. Cool the mixture to room temperature and transfer it to a 125-mL separatory funnel. 6. Extract the reaction mixture with two 20mL portions of diethyl ether. Remember the diethyl ether layer should be on top! 7. Combine the ether extracts and dry them over anhydrous MgSO4. 8. Filter off the drying agent by vacuum filtration with a Bchner funnel (see setup in Part 1) and transfer the filtrate to a 100 -mL round bottom flask. 9. Concentrate the filtrate using a rotary evaporator. 10. Cool the concentrated filtrate in an ice bath and scratch the flask with a glass pipette or metal spatula until crystallization occurs. 11. Collect the crystals by vacuum filtration with a Bchner funnel (see setup in Part 1) and allow the vacuum to dry the crystals by pulling air through them. Based on your data, fill in the correct products in the reaction scheme below. EtOHNaBH4 4. Based on the limiting reagent and product molecular weights, what are the theoretical yields of each of the reduction products? Show your work. See the YouTube video on limiting reagents and theoretical yields for help: Limiting Reagents and Percent Yield Based on the weights of your products and the theoretical yield, what is the percent yield of the two reactions of your team? Show your work. \% Yield from Part 1: % Yield from Part 2: 6. Calculate the average weights of products from Parts 1 and 2 from the other 11 Teams of your lab. Put the data in the Average column in Table 3. Table 3. Weights of reduction products formed (in grams) 7. What is the overall percent yield of the reactions based on the average class weights? Average \% Yield from Part 1: Average \% Yield from Part 2: 8. What reagent(s) could be used to reduce both the nitro and ketone functional groups in a single reaction? Procedure Part 1 - Reduction using tin and hydrochloric acid a) Reaction 1. Place small pieces of tin (3.3g) in a 100mL round bottom flask equipped with a reflux condenser and a magnetic stir bar. 2. Add 3 -nitroacetophenone (1.65g) to the flask, followed by water (24mL) and concentrated HCl(9mL). Concentrated hydrochloric acid causes severe bums. 3. Stir the mixture, and heat the flask to reflux for one hour While this reaction is proceeding, obtain an IR spectrum of the starting material, 3-nitroacetophenone b) Isolation of Crude Product 4. Cool the reaction mixture to room temperature and remove the unreacted tin by suction filtration with a Bchner funnel. (While the reaction is cooling you should start heating Part 2 - Reduction using sodium borohydride a) Reaction 1. Dissolve 3-nitroacetophenone (1.65g) in warm 100% ethanol (20mL) in a 125mL Erlenmeyer flask. 2. Stir the solution using a magnetic stirrer, and cool the flask in an ice bath (the starting material may form a fine precipitate at this point). 3. Add the sodium borohydride (0.45g) in small portions over 5min and stir the reaction mixture at room temperature for 15min. 4. Add water (15mL) to the mixture, nd heat it to its boiling point. b) Isolation of Product 5. Cool the mixture to room temperature and transfer it to a 125-mL separatory funnel. 6. Extract the reaction mixture with two 20mL portions of diethyl ether. Remember the diethyl ether layer should be on top! 7. Combine the ether extracts and dry them over anhydrous MgSO4. 8. Filter off the drying agent by vacuum filtration with a Bchner funnel (see setup in Part 1) and transfer the filtrate to a 100 -mL round bottom flask. 9. Concentrate the filtrate using a rotary evaporator. 10. Cool the concentrated filtrate in an ice bath and scratch the flask with a glass pipette or metal spatula until crystallization occurs. 11. Collect the crystals by vacuum filtration with a Bchner funnel (see setup in Part 1) and allow the vacuum to dry the crystals by pulling air through them. Based on your data, fill in the correct products in the reaction scheme below. EtOHNaBH4 4. Based on the limiting reagent and product molecular weights, what are the theoretical yields of each of the reduction products? Show your work. See the YouTube video on limiting reagents and theoretical yields for help: Limiting Reagents and Percent Yield Based on the weights of your products and the theoretical yield, what is the percent yield of the two reactions of your team? Show your work. \% Yield from Part 1: % Yield from Part 2: 6. Calculate the average weights of products from Parts 1 and 2 from the other 11 Teams of your lab. Put the data in the Average column in Table 3. Table 3. Weights of reduction products formed (in grams) 7. What is the overall percent yield of the reactions based on the average class weights? Average \% Yield from Part 1: Average \% Yield from Part 2: 8. What reagent(s) could be used to reduce both the nitro and ketone functional groups in a single reaction Picture on the bottom will give you values. 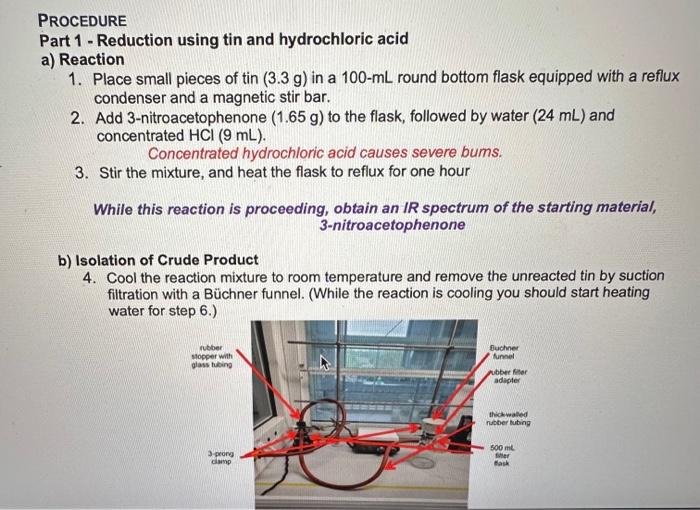




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


