Can you help me with question 4?
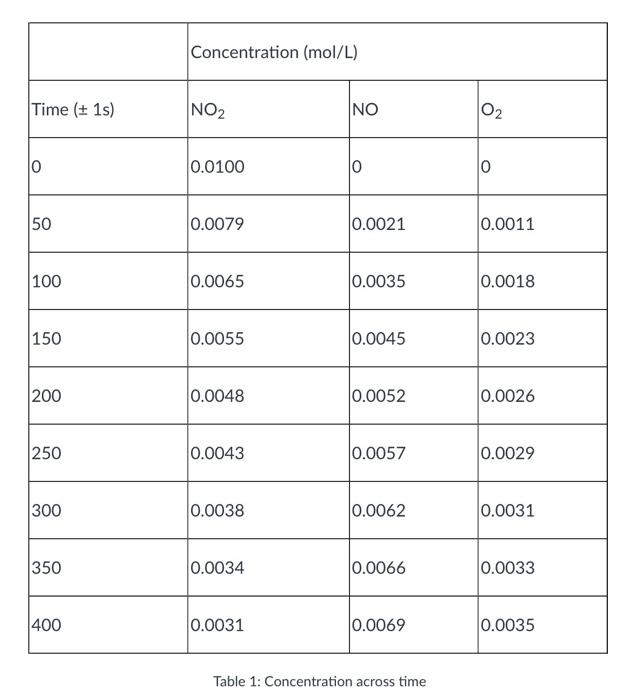
Nitrogen dioxide, a gas that causes air pollution, decomposes into nitric oxide and oxygen according to the following equation: 2NO2()2NO(g) + O2(8). Suppose in an experiment we start with a flask of nitrogen dioxide at room temperature and quickly heat it up to 300C, where it decomposes according to the above equation. We then measure the concentration of nitrogen dioxide, nitric oxide, and oxygen over time as the nitrogen dioxide decomposes. The results of this experiment are summarized in the table below. Using this data, answer the questions below. Concentration (mol/L) Time (+ 1s) NO2 INO 02 0 0.0100 0 0 50 0.0079 0.0021 0.0011 100 0.0065 0.0035 0.0018 150 0.0055 0.0045 0.0023 200 0.0048 0.0052 0.0026 250 0.0043 0.0057 0.0029 300 0.0038 0.0062 0.0031 350 0.0034 0.0066 0.0033 400 0.0031 0.0069 0.0035 Table 1: Concentration across time 1. Using the data above given on Table 1, create a data plot with time on the x-axis and concentration of chemicals on the y-axis. You will have three graphs on the same plot, one for each of the involved chemicals. Be sure to label and title your graph appropriately. Check the guides on how to create a plot using Excel or Google Sheets. 2. Draw a line connecting the points (0,0.0100) and (400, 0.0031) on the graph of NO2 concentration over time. What is the slope of this line? What are the units? What does it represent? Note: In the language of chemistry, this question is asking you to compute the average rate at which the concentration of NO2 changes during the first 400 seconds of the reaction. 3. Given that chemical reaction rates tend to slow down over time, do you expect the instantaneous change in concentration of NO when t=0 to be greater than, less than or equal to the slope computed in number 2? Similarly, do you expect the instantaneous change of concentration of NO at t=400 to be greater than, less than or equal to the slope computed in number 2? Explain your reasoning. 4. Use the data and your plot to estimate the instantaneous rate of change of concentration of NO2, NO, and O2 when t=100. What are the units for these numbers that are calculated? What do these values represent in the context of this problem? Note: In the language of chemistry, this question is asking you to estimate the instantaneous reaction rate of NO2, NO, and O2 100 seconds into the reaction