Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can you just show me one iteration so that I can see if I am on the right track? Thank you! Table 3-1 Appendix D
Can you just show me one iteration so that I can see if I am on the right track? Thank you!
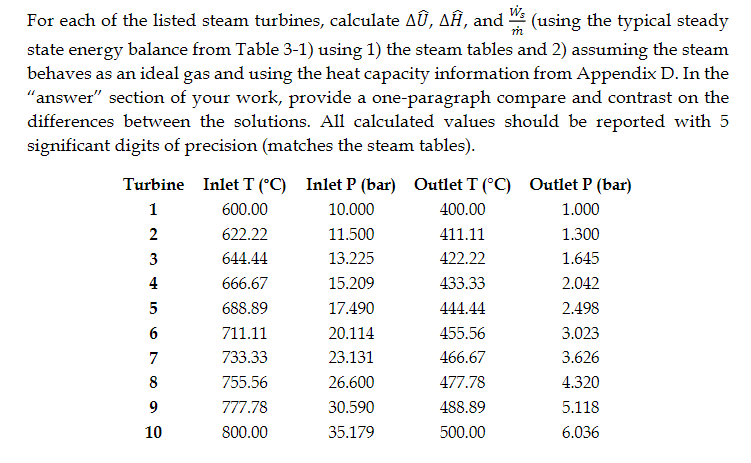
Table 3-1
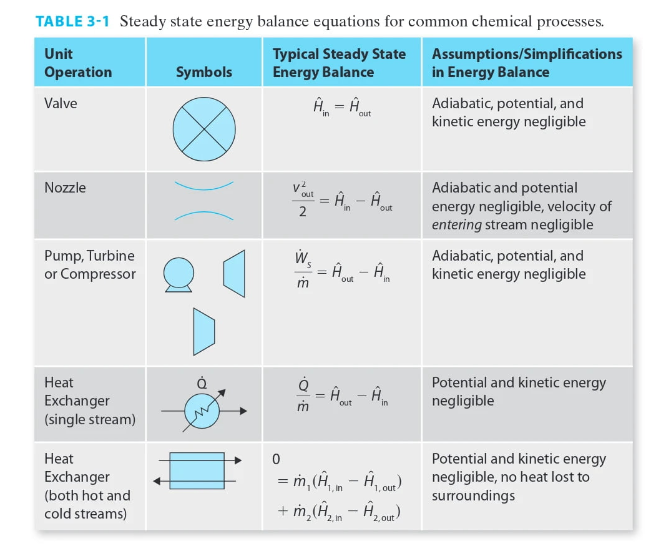
Appendix D
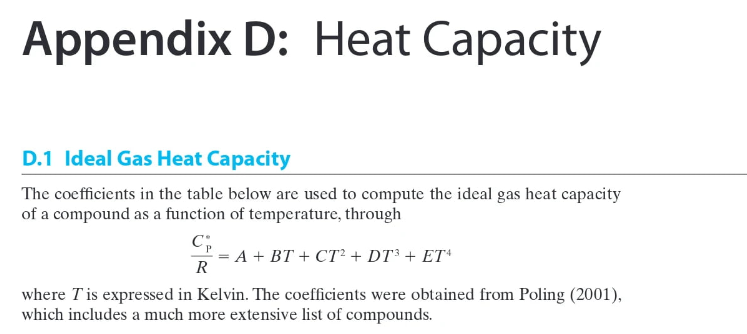
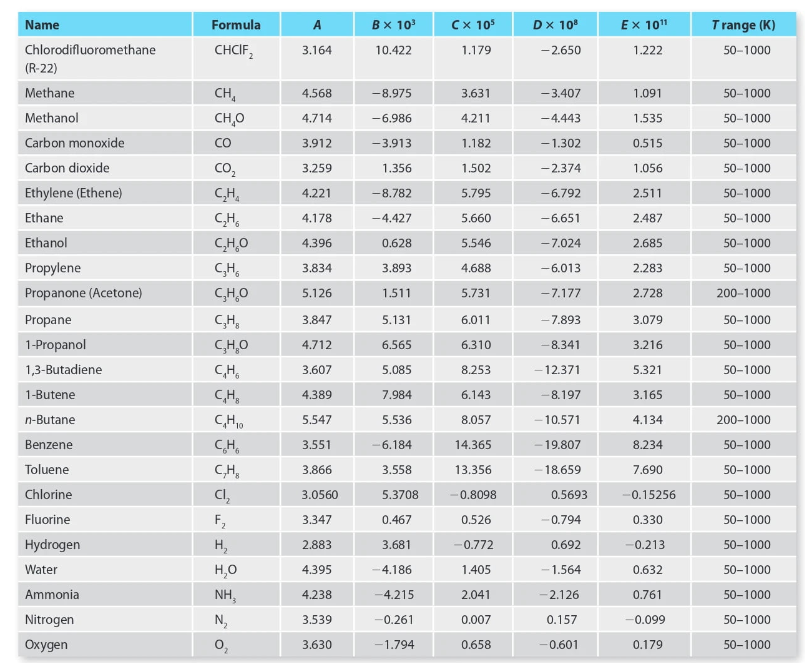
For each of the listed steam turbines, calculate All, A, and ws (using the typical steady state energy balance from Table 3-1) using 1) the steam tables and 2) assuming the steam behaves as an ideal gas and using the heat capacity information from Appendix D. In the "answer" section of your work, provide a one-paragraph compare and contrast on the differences between the solutions. All calculated values should be reported with 5 significant digits of precision (matches the steam tables). Turbine Inlet T (C) Inlet P (bar) Outlet T (C) Outlet P (bar) 1 600.00 10.000 400.00 1.000 2 622.22 11.500 411.11 1.300 3 644.44 13.225 422.22 1.645 4 666.67 15.209 433.33 2.042 5 688.89 17.490 444.44 2.498 6 711.11 20.114 455.56 3.023 7 733.33 23.131 466.67 3.626 8 755.56 26.600 477.78 4.320 9 777.78 30.590 488.89 5.118 10 800.00 35.179 500.00 6.036 9 TABLE 3-1 Steady state energy balance equations for common chemical processes. Unit Typical Steady State Assumptions/Simplifications Operation Symbols Energy Balance in Energy Balance Valve = Adiabatic potential, and kinetic energy negligible in out Nozzle v2 Out = An - Hour = 2. in Adiabatic and potential energy negligible, velocity of entering stream negligible Adiabatic, potential, and kinetic energy negligible Pump, Turbine or Compressor - 1 out in Heat Exchanger (single stream) = our - A = m Potential and kinetic energy negligible in Heat Exchanger (both hot and cold streams) Potential and kinetic energy negligible, no heat lost to surroundings 1. = m, (In - CUP) + m2 (in - Azout) 1, out 2. 2. Appendix D: Heat Capacity D.1 Ideal Gas Heat Capacity The coefficients in the table below are used to compute the ideal gas heat capacity of a compound as a function of temperature, through = A + BT + CT? + DT + ET4 R where T is expressed in Kelvin. The coefficients were obtained from Poling (2001), which includes a much more extensive list of compounds. A Formula CHCIF BX 10% 10.422 CX 105 1.179 DX 10% -2.650 EX 10" 1.222 Trange (K) 50-1000 3.164 4.568 -8.975 -3.407 CH, CH,0 3.631 4.211 4.714 -6.986 -4.443 1.091 1.535 0.515 1.056 CO 3.912 -3.913 1.182 - 1.302 3.259 1.356 1.502 5.795 -2.374 -6.792 4.221 -8.782 2.511 50-1000 50-1000 50-1000 50-1000 50-1000 50-1000 50-1000 50-1000 200-1000 4.178 -4.427 5.660 -6.651 2.487 4.396 0.628 5.546 - 7.024 2.685 CO CH CH CHO CH CHO CH CH,0 3.834 3.893 4.688 2.283 -6.013 - 7.177 5.126 1.511 5.731 2.728 3.847 Name Chlorodifluoromethane (R-22) Methane Methanol Carbon monoxide Carbon dioxide Ethylene (Ethene) Ethane Ethanol Propylene Propanone (Acetone) Propane 1-Propanol 1,3-Butadiene 1-Butene n-Butane Benzene Toluene Chlorine Fluorine Hydrogen Water Ammonia Nitrogen Oxygen 6.011 4.712 6.310 5.131 6.565 5.085 7.984 5.536 3.079 3.216 5.321 3.165 3.607 8.253 CHE 4.389 6.143 CHO 5.547 8.057 4.134 3.551 --6.184 14.365 8.234 CH CH, - 7.893 -8.341 -12.371 -8.197 -10.571 - 19.807 18.659 0.5693 -0.794 0.692 -1.564 -2.126 50-1000 50-1000 50-1000 50-1000 200-1000 50-1000 50-1000 50-1000 50-1000 50-1000 50-1000 50-1000 3.866 13.356 7.690 3.558 5.3708 ch -0.8098 3.0560 3.347 2.883 0.467 F: H, HO NH N, 3.681 -0.15256 0.330 -0.213 0.632 0.526 -0.772 1.405 4.395 4.186 4.238 -4.215 2.041 3.539 -0.261 0.007 0.157 0.761 -0.099 0.179 50-1000 3.630 -1.794 0.658 0.601 50-1000 For each of the listed steam turbines, calculate All, A, and ws (using the typical steady state energy balance from Table 3-1) using 1) the steam tables and 2) assuming the steam behaves as an ideal gas and using the heat capacity information from Appendix D. In the "answer" section of your work, provide a one-paragraph compare and contrast on the differences between the solutions. All calculated values should be reported with 5 significant digits of precision (matches the steam tables). Turbine Inlet T (C) Inlet P (bar) Outlet T (C) Outlet P (bar) 1 600.00 10.000 400.00 1.000 2 622.22 11.500 411.11 1.300 3 644.44 13.225 422.22 1.645 4 666.67 15.209 433.33 2.042 5 688.89 17.490 444.44 2.498 6 711.11 20.114 455.56 3.023 7 733.33 23.131 466.67 3.626 8 755.56 26.600 477.78 4.320 9 777.78 30.590 488.89 5.118 10 800.00 35.179 500.00 6.036 9 TABLE 3-1 Steady state energy balance equations for common chemical processes. Unit Typical Steady State Assumptions/Simplifications Operation Symbols Energy Balance in Energy Balance Valve = Adiabatic potential, and kinetic energy negligible in out Nozzle v2 Out = An - Hour = 2. in Adiabatic and potential energy negligible, velocity of entering stream negligible Adiabatic, potential, and kinetic energy negligible Pump, Turbine or Compressor - 1 out in Heat Exchanger (single stream) = our - A = m Potential and kinetic energy negligible in Heat Exchanger (both hot and cold streams) Potential and kinetic energy negligible, no heat lost to surroundings 1. = m, (In - CUP) + m2 (in - Azout) 1, out 2. 2. Appendix D: Heat Capacity D.1 Ideal Gas Heat Capacity The coefficients in the table below are used to compute the ideal gas heat capacity of a compound as a function of temperature, through = A + BT + CT? + DT + ET4 R where T is expressed in Kelvin. The coefficients were obtained from Poling (2001), which includes a much more extensive list of compounds. A Formula CHCIF BX 10% 10.422 CX 105 1.179 DX 10% -2.650 EX 10" 1.222 Trange (K) 50-1000 3.164 4.568 -8.975 -3.407 CH, CH,0 3.631 4.211 4.714 -6.986 -4.443 1.091 1.535 0.515 1.056 CO 3.912 -3.913 1.182 - 1.302 3.259 1.356 1.502 5.795 -2.374 -6.792 4.221 -8.782 2.511 50-1000 50-1000 50-1000 50-1000 50-1000 50-1000 50-1000 50-1000 200-1000 4.178 -4.427 5.660 -6.651 2.487 4.396 0.628 5.546 - 7.024 2.685 CO CH CH CHO CH CHO CH CH,0 3.834 3.893 4.688 2.283 -6.013 - 7.177 5.126 1.511 5.731 2.728 3.847 Name Chlorodifluoromethane (R-22) Methane Methanol Carbon monoxide Carbon dioxide Ethylene (Ethene) Ethane Ethanol Propylene Propanone (Acetone) Propane 1-Propanol 1,3-Butadiene 1-Butene n-Butane Benzene Toluene Chlorine Fluorine Hydrogen Water Ammonia Nitrogen Oxygen 6.011 4.712 6.310 5.131 6.565 5.085 7.984 5.536 3.079 3.216 5.321 3.165 3.607 8.253 CHE 4.389 6.143 CHO 5.547 8.057 4.134 3.551 --6.184 14.365 8.234 CH CH, - 7.893 -8.341 -12.371 -8.197 -10.571 - 19.807 18.659 0.5693 -0.794 0.692 -1.564 -2.126 50-1000 50-1000 50-1000 50-1000 200-1000 50-1000 50-1000 50-1000 50-1000 50-1000 50-1000 50-1000 3.866 13.356 7.690 3.558 5.3708 ch -0.8098 3.0560 3.347 2.883 0.467 F: H, HO NH N, 3.681 -0.15256 0.330 -0.213 0.632 0.526 -0.772 1.405 4.395 4.186 4.238 -4.215 2.041 3.539 -0.261 0.007 0.157 0.761 -0.099 0.179 50-1000 3.630 -1.794 0.658 0.601 50-1000
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


