Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Can you please answer ,4,and 5 and 6 and provide step by step Problem 3 (25 points) Consider the Txy and Pxy vapor liquid equilihri
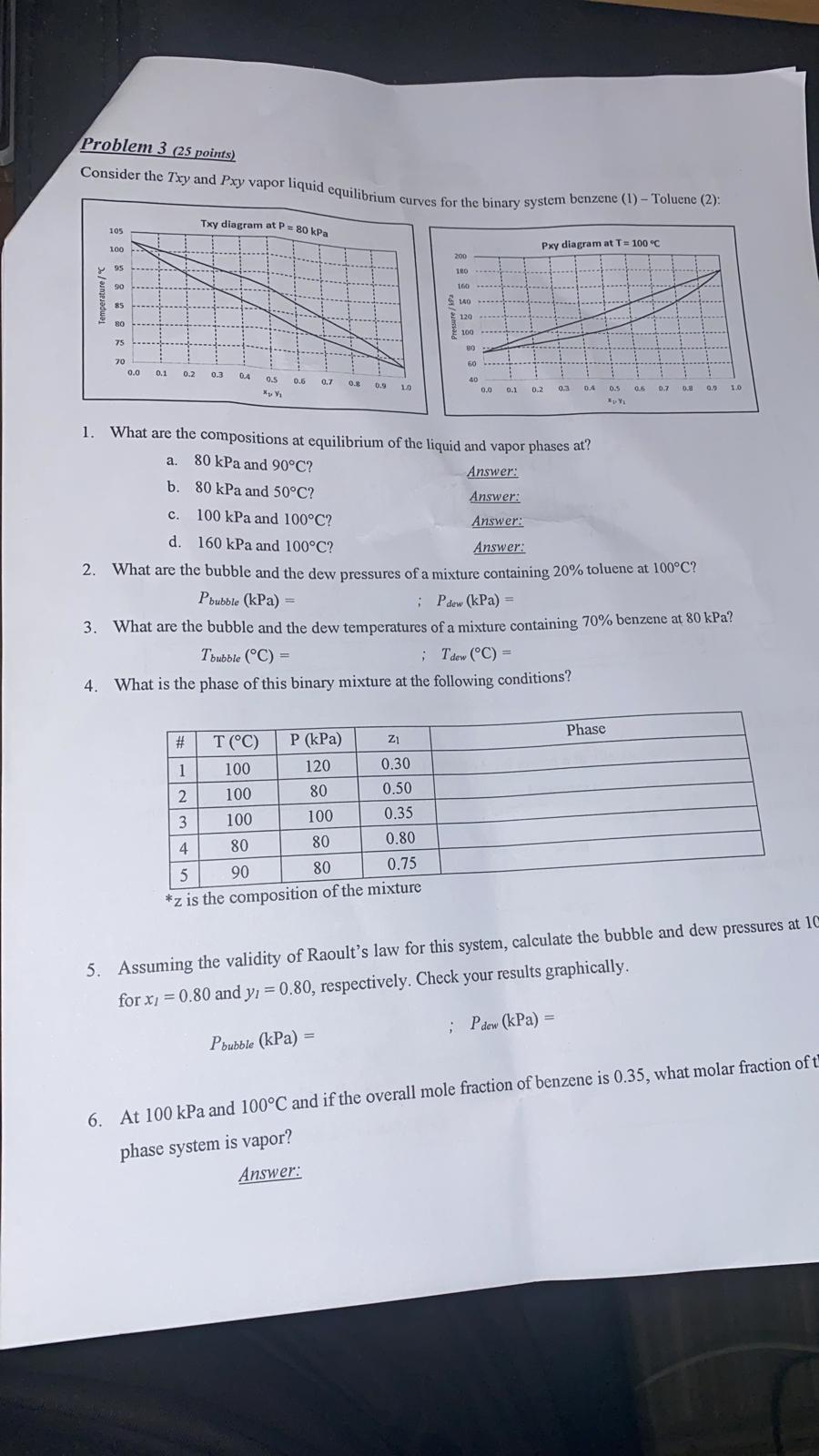
Can you please answer ,4,and 5 and 6 and provide step by step
Problem 3 (25 points) Consider the Txy and Pxy vapor liquid equilihri les for the binary system benzene (1) - Toluene (2): 1. What are the compositions at equilibrium of the liquid and vapor phases at? a. 80kPa and 90C ? Answer: b. 80kPa and 50C ? Answer: c. 100kPa and 100C ? Answer: d. 160kPa and 100C ? Answer: 2. What are the bubble and the dew pressures of a mixture containing 20% toluene at 100C ? Pbubble(kPa)=;Pdew(kPa)= 3. What are the bubble and the dew temperatures of a mixture containing 70% benzene at 80kPa ? Tbubble(C)=;Tdew(C)= 4. What is the phase of this binary mixture at the following conditions? 5. Assuming the validity of Raoult's law for this system, calculate the bubble and dew pressures at 10 for x1=0.80 and y1=0.80, respectively. Check your results graphically. Pbubble(kPa)= 6. At 100kPa and 100C and if the overall mole fraction of benzene is 0.35, what molar fraction of t phase system is vaporStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


